Lentivirus-mediated Gene Transfer in Hematopoietic Stem Cells Is Impaired in SHIV-infected, ART-treated Nonhuman Primates
- PMID: 25648264
- PMCID: PMC4427875
- DOI: 10.1038/mt.2015.19
Lentivirus-mediated Gene Transfer in Hematopoietic Stem Cells Is Impaired in SHIV-infected, ART-treated Nonhuman Primates
Abstract
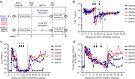
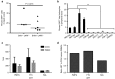
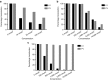
Recent studies have demonstrated that genetically modified hematopoietic stem cells (HSCs) can reduce HIV viremia. We have developed an HIV/AIDS-patient model in Simian/human immunodeficiency virus (SHIV)-infected pigtailed macaques that are stably suppressed on antiretroviral therapy (ART: raltegravir, emtricitabine and tenofovir). Following SHIV infection and ART, animals undergo autologous HSC transplantation (HSCT) with lentivirally transduced cluster of differentiation (CD)34(+) cells expressing the mC46 anti-HIV fusion protein. We show that SHIV(+), ART-treated animals had very low gene marking levels after HSCT. Pretransduction CD34(+) cells contained detectable levels of all three ART drugs, likely contributing to the low gene transfer efficiency. Following HSCT recovery and the cessation of ART, plasma viremia rebounded, indicating that myeloablative total body irradiation cannot completely eliminate viral reservoirs after autologous HSCT. The kinetics of recovery following autologous HSCT in SHIV(+), ART-treated macaques paralleled those observed following transplantation of control animals. However, T-cell subset analyses demonstrated a high percentage of C-C chemokine receptor 5 (CCR5)-expressing CD4(+) T-cells after HSCT. These data suggest that an extended ART interruption time may be required for more efficient lentiviral transduction. To avoid complications associated with ART interruption in the context of high percentages of CD4(+)CCR5(+)T-cells after HSCT, the use of vector systems not impaired by the presence of residual ART may also be beneficial.
Figures

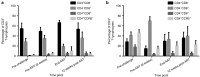
Similar articles
-
Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant.PLoS Pathog. 2014 Sep 25;10(9):e1004406. doi: 10.1371/journal.ppat.1004406. eCollection 2014 Sep. PLoS Pathog. 2014. PMID: 25254512 Free PMC article.
-
Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model.Blood. 2013 Jul 11;122(2):179-87. doi: 10.1182/blood-2013-01-482224. Epub 2013 May 29. Blood. 2013. PMID: 23719296 Free PMC article.
-
Combinatorial hematopoietic stem cell transplantation and vaccination reduces viral pathogenesis following SHIV89.6P-challenge.Gene Ther. 2015 Dec;22(12):1007-12. doi: 10.1038/gt.2015.83. Epub 2015 Sep 10. Gene Ther. 2015. PMID: 26355737 Free PMC article.
-
In Vivo Hematopoietic Stem Cell Transduction.Hematol Oncol Clin North Am. 2017 Oct;31(5):771-785. doi: 10.1016/j.hoc.2017.06.001. Hematol Oncol Clin North Am. 2017. PMID: 28895846 Free PMC article. Review.
-
Genetically modified hematopoietic stem cell transplantation for HIV-1-infected patients: can we achieve a cure?Mol Ther. 2014 Feb;22(2):257-264. doi: 10.1038/mt.2013.264. Epub 2013 Nov 13. Mol Ther. 2014. PMID: 24220323 Free PMC article. Review.
Cited by
-
Stem cell-based therapies for HIV/AIDS.Adv Drug Deliv Rev. 2016 Aug 1;103:187-201. doi: 10.1016/j.addr.2016.04.027. Epub 2016 May 2. Adv Drug Deliv Rev. 2016. PMID: 27151309 Free PMC article. Review.
-
Autologous, Gene-Modified Hematopoietic Stem and Progenitor Cells Repopulate the Central Nervous System with Distinct Clonal Variants.Stem Cell Reports. 2019 Jul 9;13(1):91-104. doi: 10.1016/j.stemcr.2019.05.016. Epub 2019 Jun 13. Stem Cell Reports. 2019. PMID: 31204301 Free PMC article.
-
Assessment and streamlined preparation of low-cytotoxicity lentiviral vectors for mobilized human hematopoietic stem cell transduction.Exp Hematol. 2020 Jun;86:28-42.e3. doi: 10.1016/j.exphem.2020.05.009. Epub 2020 May 27. Exp Hematol. 2020. PMID: 32473295 Free PMC article.
-
The clonal repopulation of HSPC gene modified with anti-HIV-1 RNAi is not affected by preexisting HIV-1 infection.Sci Adv. 2020 Jul 22;6(30):eaay9206. doi: 10.1126/sciadv.aay9206. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32766447 Free PMC article.
-
Multilineage polyclonal engraftment of Cal-1 gene-modified cells and in vivo selection after SHIV infection in a nonhuman primate model of AIDS.Mol Ther Methods Clin Dev. 2016 Feb 24;3:16007. doi: 10.1038/mtm.2016.7. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 26958575 Free PMC article.
References
-
- Little RF, Dunleavy K. Update on the treatment of HIV-associated hematologic malignancies. Hematology Am Soc Hematol Educ Program. 2013;2013:382–388. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

