Effect of pantoprazole to enhance activity of docetaxel against human tumour xenografts by inhibiting autophagy
- PMID: 25647012
- PMCID: PMC4453951
- DOI: 10.1038/bjc.2015.17
Effect of pantoprazole to enhance activity of docetaxel against human tumour xenografts by inhibiting autophagy
Retraction in
-
Retraction Note: Effect of pantoprazole to enhance activity of docetaxel against human tumour xenografts by inhibiting autophagy.Br J Cancer. 2024 Apr;130(7):1232. doi: 10.1038/s41416-024-02660-4. Br J Cancer. 2024. PMID: 38509357 No abstract available.
Abstract
Background: Autophagy allows recycling of cellular components and may facilitate cell survival after chemotherapy. Pantoprazole inhibits proton pumps and is reported to inhibit autophagy. Here we evaluate the effects of pantoprazole to modify cytotoxicity of the anticancer drug docetaxel, and underlying mechanisms.
Methods: Effects of docetaxel±pantoprazole were studied against wild-type and autophagy-deficient PC3 cells and against four human xenografts. Effects of pantoprazole on autophagy were evaluated by quantifying LC3-I, LC3-II and p62 proteins in western blots, and by fluorescent microscopy of cells transfected with RFP-GFP-LC3. The distribution of drug effects and of autophagy was quantified in tumour sections in relation to blood vessels and hypoxia by immunohistochemistry using γH2AX, cleaved caspase-3, Ki67 and LC3/ p62.
Results: Pantoprazole increased the toxicity of docetaxel in vitro, increased docetaxel-induced expression of γH2AX and cleaved caspase-3, and decreased Ki67 in tumour sections. Pantoprazole increased growth delay of four human xenografts of low, moderate and high sensitivity to docetaxel, with minimal increase in toxicity. Docetaxel led to increased autophagy throughout tumour sections. Pantoprazole inhibited autophagy, and effects of pantoprazole were reduced against genetically modified cells with decreased ability to undergo autophagy.
Conclusions: Autophagy is a mechanism of resistance to docetaxel chemotherapy that may be modified by pantoprazole to improve therapeutic index.
Figures
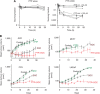
 ,500 μ
,500 μ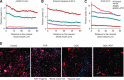
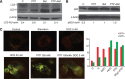
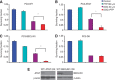
Similar articles
-
Up-regulation of autophagy is a mechanism of resistance to chemotherapy and can be inhibited by pantoprazole to increase drug sensitivity.Cancer Chemother Pharmacol. 2017 May;79(5):959-969. doi: 10.1007/s00280-017-3298-5. Epub 2017 Apr 4. Cancer Chemother Pharmacol. 2017. PMID: 28378028
-
Role of Autophagy as a Survival Mechanism for Hypoxic Cells in Tumors.Neoplasia. 2016 Jun;18(6):347-55. doi: 10.1016/j.neo.2016.04.003. Neoplasia. 2016. Retraction in: Neoplasia. 2024 May;51:100994. doi: 10.1016/j.neo.2024.100994 PMID: 27292024 Free PMC article. Retracted.
-
Activity of the hypoxia-activated pro-drug TH-302 in hypoxic and perivascular regions of solid tumors and its potential to enhance therapeutic effects of chemotherapy.Int J Cancer. 2014 Jun 1;134(11):2726-34. doi: 10.1002/ijc.28595. Epub 2013 Dec 13. Int J Cancer. 2014. PMID: 24338277
-
Pantoprazole Affecting Docetaxel Resistance Pathways via Autophagy (PANDORA): Phase II Trial of High Dose Pantoprazole (Autophagy Inhibitor) with Docetaxel in Metastatic Castration-Resistant Prostate Cancer (mCRPC).Oncologist. 2019 Sep;24(9):1188-1194. doi: 10.1634/theoncologist.2018-0621. Epub 2019 Apr 5. Oncologist. 2019. PMID: 30952818 Free PMC article. Clinical Trial.
-
Use of the proton pump inhibitor pantoprazole to modify the distribution and activity of doxorubicin: a potential strategy to improve the therapy of solid tumors.Clin Cancer Res. 2013 Dec 15;19(24):6766-76. doi: 10.1158/1078-0432.CCR-13-0128. Epub 2013 Oct 18. Clin Cancer Res. 2013. PMID: 24141627
Cited by
-
Docetaxel enhances lysosomal function through TFEB activation.Cell Death Dis. 2018 May 23;9(6):614. doi: 10.1038/s41419-018-0571-4. Cell Death Dis. 2018. PMID: 29795139 Free PMC article.
-
Establishment and large-scale validation of a three-dimensional tumor model on an array chip for anticancer drug evaluation.Front Pharmacol. 2022 Oct 12;13:1032975. doi: 10.3389/fphar.2022.1032975. eCollection 2022. Front Pharmacol. 2022. PMID: 36313330 Free PMC article.
-
CCL2-SQSTM1 positive feedback loop suppresses autophagy to promote chemoresistance in gastric cancer.Int J Biol Sci. 2018 Jun 3;14(9):1054-1066. doi: 10.7150/ijbs.25349. eCollection 2018. Int J Biol Sci. 2018. PMID: 29989092 Free PMC article.
-
Influence of the proton pump inhibitor lansoprazole on distribution and activity of doxorubicin in solid tumors.Cancer Sci. 2015 Oct;106(10):1438-47. doi: 10.1111/cas.12756. Epub 2015 Sep 25. Cancer Sci. 2015. PMID: 26212113 Free PMC article.
-
Blockage of Autophagy for Cancer Therapy: A Comprehensive Review.Int J Mol Sci. 2024 Jul 7;25(13):7459. doi: 10.3390/ijms25137459. Int J Mol Sci. 2024. PMID: 39000565 Free PMC article. Review.
References
-
- Bardag-Gorce F, Francis T, Nan L, Li J, He Lue Y, French BA, French SW. Modifications in P62 occur due to proteasome inhibition in alcoholic liver disease. Life Sci. 2005;77:2594–2602. - PubMed
-
- Bradley G, Ling V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994;13:223–233. - PubMed
-
- Brana I, Ocana A, Chen EX, Razak AR, Haines C, Lee C, Douglas S, Wang L, Siu LL, Tannock IF, Bedard PL. A phase I trial of pantoprazole in combination with doxorubicin in patients with advanced solid tumors: evaluation of pharmacokinetics of both drugs and tissue penetration of doxorubicin. Invest New Drugs. 2014;32:1269–1277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

