IFN regulatory factor 8 represses GM-CSF expression in T cells to affect myeloid cell lineage differentiation
- PMID: 25646302
- PMCID: PMC4340766
- DOI: 10.4049/jimmunol.1402412
IFN regulatory factor 8 represses GM-CSF expression in T cells to affect myeloid cell lineage differentiation
Abstract
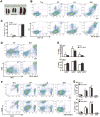
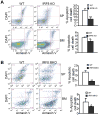
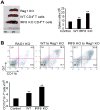
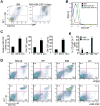
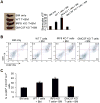
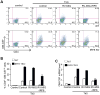
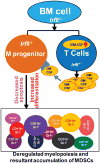
During hematopoiesis, hematopoietic stem cells constantly differentiate into granulocytes and macrophages via a distinct differentiation program that is tightly controlled by myeloid lineage-specific transcription factors. Mice with a null mutation of IFN regulatory factor 8 (IRF8) accumulate CD11b(+)Gr1(+) myeloid cells that phenotypically and functionally resemble tumor-induced myeloid-derived suppressor cells (MDSCs), indicating an essential role of IRF8 in myeloid cell lineage differentiation. However, IRF8 is expressed in various types of immune cells, and whether IRF8 functions intrinsically or extrinsically in regulation of myeloid cell lineage differentiation is not fully understood. In this study, we report an intriguing finding that, although IRF8-deficient mice exhibit deregulated myeloid cell differentiation and resultant accumulation of CD11b(+)Gr1(+) MDSCs, surprisingly, mice with IRF8 deficiency only in myeloid cells exhibit no abnormal myeloid cell lineage differentiation. Instead, mice with IRF8 deficiency only in T cells exhibited deregulated myeloid cell differentiation and MDSC accumulation. We further demonstrated that IRF8-deficient T cells exhibit elevated GM-CSF expression and secretion. Treatment of mice with GM-CSF increased MDSC accumulation, and adoptive transfer of IRF8-deficient T cells, but not GM-CSF-deficient T cells, increased MDSC accumulation in the recipient chimeric mice. Moreover, overexpression of IRF8 decreased GM-CSF expression in T cells. Our data determine that, in addition to its intrinsic function as an apoptosis regulator in myeloid cells, IRF8 also acts extrinsically to repress GM-CSF expression in T cells to control myeloid cell lineage differentiation, revealing a novel mechanism that the adaptive immune component of the immune system regulates the innate immune cell myelopoiesis in vivo.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
Conflict of interests: None
Figures


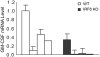
Similar articles
-
A reporter mouse reveals lineage-specific and heterogeneous expression of IRF8 during lymphoid and myeloid cell differentiation.J Immunol. 2014 Aug 15;193(4):1766-77. doi: 10.4049/jimmunol.1301939. Epub 2014 Jul 14. J Immunol. 2014. PMID: 25024380 Free PMC article.
-
The Granulocyte Progenitor Stage Is a Key Target of IRF8-Mediated Regulation of Myeloid-Derived Suppressor Cell Production.J Immunol. 2017 May 15;198(10):4129-4139. doi: 10.4049/jimmunol.1601722. Epub 2017 Mar 29. J Immunol. 2017. PMID: 28356386 Free PMC article.
-
Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF.Eur J Immunol. 2010 Jan;40(1):22-35. doi: 10.1002/eji.200939903. Eur J Immunol. 2010. PMID: 19941314
-
Mechanisms overseeing myeloid-derived suppressor cell production in neoplastic disease.Cancer Immunol Immunother. 2017 Aug;66(8):989-996. doi: 10.1007/s00262-017-1963-5. Epub 2017 Feb 21. Cancer Immunol Immunother. 2017. PMID: 28224211 Free PMC article. Review.
-
GM-CSF: An immune modulatory cytokine that can suppress autoimmunity.Cytokine. 2015 Oct;75(2):261-71. doi: 10.1016/j.cyto.2015.05.030. Epub 2015 Jun 22. Cytokine. 2015. PMID: 26113402 Free PMC article. Review.
Cited by
-
Th17 cell differentiation proceeds independently of IRF8.Immunol Cell Biol. 2016 Sep;94(8):796-801. doi: 10.1038/icb.2016.33. Epub 2016 May 3. Immunol Cell Biol. 2016. PMID: 27140932
-
Granulocytic Myeloid-Derived Suppressor Cells as Negative Regulators of Anticancer Immunity.Front Immunol. 2020 Aug 27;11:1963. doi: 10.3389/fimmu.2020.01963. eCollection 2020. Front Immunol. 2020. PMID: 32983128 Free PMC article. Review.
-
Special Conference on Tumor Immunology and Immunotherapy: A New Chapter.Cancer Immunol Res. 2015 Jun;3(6):590-597. doi: 10.1158/2326-6066.CIR-15-0106. Epub 2015 May 12. Cancer Immunol Res. 2015. PMID: 25968457 Free PMC article.
-
Polarization of granulocytic myeloid-derived suppressor cells by hepatitis C core protein is mediated via IL-10/STAT3 signalling.J Viral Hepat. 2019 Feb;26(2):246-257. doi: 10.1111/jvh.13024. Epub 2018 Nov 19. J Viral Hepat. 2019. PMID: 30339295 Free PMC article.
-
TRAF3: a novel tumor suppressor gene in macrophages.Macrophage (Houst). 2015 Sep 30;2:e1009. doi: 10.14800/macrophage.1009. Macrophage (Houst). 2015. PMID: 26661944 Free PMC article.
References
-
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI091871/AI/NIAID NIH HHS/United States
- AI104688/AI/NIAID NIH HHS/United States
- R01 CA182518/CA/NCI NIH HHS/United States
- CA133085/CA/NCI NIH HHS/United States
- R01 CA190400/CA/NCI NIH HHS/United States
- DK072201/DK/NIDDK NIH HHS/United States
- CA182518/CA/NCI NIH HHS/United States
- I01 BX001962/BX/BLRD VA/United States
- R01 CA133085/CA/NCI NIH HHS/United States
- AI091871/AI/NIAID NIH HHS/United States
- Intramural NIH HHS/United States
- P01 DK072201/DK/NIDDK NIH HHS/United States
- CA140622/CA/NCI NIH HHS/United States
- R01 CA140622/CA/NCI NIH HHS/United States
- R01 CA158202/CA/NCI NIH HHS/United States
- U01 AI095611/AI/NIAID NIH HHS/United States
- R01 AI104688/AI/NIAID NIH HHS/United States
- R21 CA185909/CA/NCI NIH HHS/United States
- R01 CA173861/CA/NCI NIH HHS/United States
- CA185909/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

