Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations
- PMID: 25645914
- PMCID: PMC4375510
- DOI: 10.1074/jbc.M114.634915
Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations
Abstract
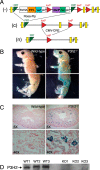
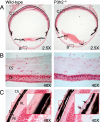
Myopia, the leading cause of visual impairment worldwide, results from an increase in the axial length of the eyeball. Mutations in LEPREL1, the gene encoding prolyl 3-hydroxylase-2 (P3H2), have recently been identified in individuals with recessively inherited nonsyndromic severe myopia. P3H2 is a member of a family of genes that includes three isoenzymes of prolyl 3-hydroxylase (P3H), P3H1, P3H2, and P3H3. Fundamentally, it is understood that P3H1 is responsible for converting proline to 3-hydroxyproline. This limited additional knowledge also suggests that each isoenzyme has evolved different collagen sequence-preferred substrate specificities. In this study, differences in prolyl 3-hydroxylation were screened in eye tissues from P3h2-null (P3h2(n/n)) and wild-type mice to seek tissue-specific effects due the lack of P3H2 activity on post-translational collagen chemistry that could explain myopia. The mice were viable and had no gross musculoskeletal phenotypes. Tissues from sclera and cornea (type I collagen) and lens capsule (type IV collagen) were dissected from mouse eyes, and multiple sites of prolyl 3-hydroxylation were identified by mass spectrometry. The level of prolyl 3-hydroxylation at multiple substrate sites from type I collagen chains was high in sclera, similar to tendon. Almost every known site of prolyl 3-hydroxylation in types I and IV collagen from P3h2(n/n) mouse eye tissues was significantly under-hydroxylated compared with their wild-type littermates. We conclude that altered collagen prolyl 3-hydroxylation is caused by loss of P3H2. We hypothesize that this leads to structural abnormalities in multiple eye tissues, but particularly sclera, causing progressive myopia.
Keywords: 3-Hydroxyproline; Animal Model; Collagen; Extracellular Matrix; Mass Spectrometry (MS); Post-translational Modification (PTM); Prolyl 3-Hydroxylase; Sclera.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
P3h3-null and Sc65-null Mice Phenocopy the Collagen Lysine Under-hydroxylation and Cross-linking Abnormality of Ehlers-Danlos Syndrome Type VIA.J Biol Chem. 2017 Mar 3;292(9):3877-3887. doi: 10.1074/jbc.M116.762245. Epub 2017 Jan 23. J Biol Chem. 2017. PMID: 28115524 Free PMC article.
-
Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification?Connect Tissue Res. 2013;54(4-5):245-51. doi: 10.3109/03008207.2013.800867. Epub 2013 Jun 21. Connect Tissue Res. 2013. PMID: 23772978 Free PMC article. Review.
-
Characterization of recombinant human prolyl 3-hydroxylase isoenzyme 2, an enzyme modifying the basement membrane collagen IV.J Biol Chem. 2008 Jul 11;283(28):19432-9. doi: 10.1074/jbc.M802973200. Epub 2008 May 15. J Biol Chem. 2008. PMID: 18487197
-
Biological role of prolyl 3-hydroxylation in type IV collagen.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):161-6. doi: 10.1073/pnas.1307597111. Epub 2013 Dec 24. Proc Natl Acad Sci U S A. 2014. PMID: 24368846 Free PMC article.
-
Role of prolyl hydroxylation in the molecular interactions of collagens.Essays Biochem. 2019 Sep 13;63(3):325-335. doi: 10.1042/EBC20180053. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31350381 Free PMC article. Review.
Cited by
-
Animal modeling for myopia.Adv Ophthalmol Pract Res. 2024 Jun 5;4(4):173-181. doi: 10.1016/j.aopr.2024.06.001. eCollection 2024 Nov-Dec. Adv Ophthalmol Pract Res. 2024. PMID: 39263386 Free PMC article. Review.
-
Age-related type I collagen modifications reveal tissue-defining differences between ligament and tendon.Matrix Biol Plus. 2021 Jun 2;12:100070. doi: 10.1016/j.mbplus.2021.100070. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34825162 Free PMC article.
-
Trio-based exome sequencing arrests de novo mutations in early-onset high myopia.Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):4219-4224. doi: 10.1073/pnas.1615970114. Epub 2017 Apr 3. Proc Natl Acad Sci U S A. 2017. PMID: 28373534 Free PMC article.
-
Reticulocalbin 3 is involved in postnatal tendon development by regulating collagen fibrillogenesis and cellular maturation.Sci Rep. 2021 May 25;11(1):10868. doi: 10.1038/s41598-021-90258-8. Sci Rep. 2021. PMID: 34035379 Free PMC article.
-
Widespread Involvement of Acetylation in the Retinal Metabolism of Form-Deprivation Myopia in Guinea Pigs.ACS Omega. 2023 Jun 26;8(26):23825-23839. doi: 10.1021/acsomega.3c02219. eCollection 2023 Jul 4. ACS Omega. 2023. PMID: 37426266 Free PMC article.
References
-
- Berg R. A., Prockop D. J. (1973) The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem. Biophys. Res. Commun. 52, 115–120 - PubMed
-
- Ogle J. D., Arlinghaus R. B., Logan M. A. (1962) 3-Hydroxyproline, a new amino acid of collagen. J. Biol. Chem. 237, 3667–3673 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD070394/HD/NICHD NIH HHS/United States
- AR37318/AR/NIAMS NIH HHS/United States
- AI036211/AI/NIAID NIH HHS/United States
- R01 AR037318/AR/NIAMS NIH HHS/United States
- P01 HD022657/HD/NICHD NIH HHS/United States
- R37 AR037318/AR/NIAMS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- R01 AR036794/AR/NIAMS NIH HHS/United States
- AR37694/AR/NIAMS NIH HHS/United States
- F32 AR063616/AR/NIAMS NIH HHS/United States
- P30 HD024064/HD/NICHD NIH HHS/United States
- HD22657/HD/NICHD NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- AR063616/AR/NIAMS NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- T32 DK060445/DK/NIDDK NIH HHS/United States
- P01 HD070394/HD/NICHD NIH HHS/United States
- RR024574/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

