Disease severity and mortality can be independently regulated in a mouse model of experimental graft versus host disease
- PMID: 25643148
- PMCID: PMC4313938
- DOI: 10.1371/journal.pone.0118079
Disease severity and mortality can be independently regulated in a mouse model of experimental graft versus host disease
Abstract
Graft versus host disease (GVHD) is the major limitation of allogeneic hematopoietic stem cell transplantation (HSCT) presenting high mortality and morbidity rates. However, the exact cause of death is not completely understood and does not correlate with specific clinical and histological parameters of disease. Here we show, by using a semi-allogeneic mouse model of GVHD, that mortality and morbidity can be experimentally separated. We injected bone marrow-derived dendritic cells (BMDC) from NOD2/CARD15-deficient donors into semi-allogeneic irradiated chimaeras and observed that recipients were protected from death. However, no protection was observed regarding clinical or pathological scores up to 20 days after transplantation. Protection from death was associated with decreased bacterial translocation, faster hematologic recovery and epithelial integrity maintenance despite mononuclear infiltration at day 20 post-GVHD induction with no skew towards different T helper phenotypes. The protected mice recovered from aGVHD and progressively reached scores compatible with healthy animals. Altogether, our data indicate that severity and mortality can be separate events providing a model to study transplant-related mortality.
Conflict of interest statement
Figures


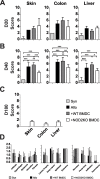
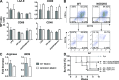
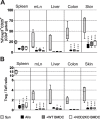
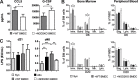
Similar articles
-
Preventive azithromycin treatment reduces noninfectious lung injury and acute graft-versus-host disease in a murine model of allogeneic hematopoietic cell transplantation.Biol Blood Marrow Transplant. 2015 Jan;21(1):30-8. doi: 10.1016/j.bbmt.2014.09.025. Epub 2014 Oct 17. Biol Blood Marrow Transplant. 2015. PMID: 25445642
-
NOD2/CARD15 single nucleotide polymorphism 13 (3020insC) is associated with risk of sepsis and single nucleotide polymorphism 8 (2104C>T) with herpes viruses reactivation in patients after allogeneic hematopoietic stem cell transplantation.Biol Blood Marrow Transplant. 2014 Mar;20(3):409-14. doi: 10.1016/j.bbmt.2013.12.558. Epub 2013 Dec 15. Biol Blood Marrow Transplant. 2014. PMID: 24345423
-
Host-derived CD8⁺ dendritic cells protect against acute graft-versus-host disease after experimental allogeneic bone marrow transplantation.Biol Blood Marrow Transplant. 2014 Nov;20(11):1696-704. doi: 10.1016/j.bbmt.2014.08.005. Epub 2014 Aug 13. Biol Blood Marrow Transplant. 2014. PMID: 25132527
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Hematopoietic stem cell graft manipulation as a mechanism of immunotherapy.Int Immunopharmacol. 2003 Aug;3(8):1121-43. doi: 10.1016/S1567-5769(03)00014-6. Int Immunopharmacol. 2003. PMID: 12860168 Review.
Cited by
-
Danger Signals and Graft-versus-host Disease: Current Understanding and Future Perspectives.Front Immunol. 2016 Nov 29;7:539. doi: 10.3389/fimmu.2016.00539. eCollection 2016. Front Immunol. 2016. PMID: 27965667 Free PMC article. Review.
References
-
- Welniak LA, Blazar BR, Murphy WJ (2007) Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 25: 139–170. - PubMed
-
- van den Brink MR, Burakoff SJ (2002) Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol 2: 273–281. - PubMed
-
- Pidala J, Kim J, Anasetti C, Nishihori T, Betts B, et al. (2011) The global severity of chronic graft-versus-host disease, determined by National Institutes of Health consensus criteria, is associated with overall survival and non-relapse mortality. Haematologica 96: 1678–1684. 10.3324/haematol.2011.049841 - DOI - PMC - PubMed
-
- Wojno KJ, Vogelsang GB, Beschorner WE, Santos GW (1994) Pulmonary hemorrhage as a cause of death in allogeneic bone marrow recipients with severe acute graft-versus-host disease. Transplantation 57: 88–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

