Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice
- PMID: 25641256
- PMCID: PMC4657468
- DOI: 10.1002/hep.27694
Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice
Abstract
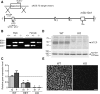
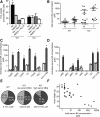
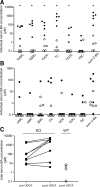
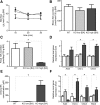
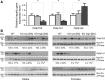
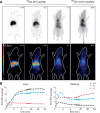
The Na(+) -taurocholate cotransporting polypeptide (NTCP) mediates uptake of conjugated bile acids (BAs) and is localized at the basolateral membrane of hepatocytes. It has recently been recognized as the receptor mediating hepatocyte-specific entry of hepatitis B virus and hepatitis delta virus. Myrcludex B, a peptide inhibitor of hepatitis B virus entry, is assumed to specifically target NTCP. Here, we investigated BA transport and Myrcludex B binding in the first Slc10a1-knockout mouse model (Slc10a1 encodes NTCP). Primary Slc10a1(-/-) hepatocytes showed absence of sodium-dependent taurocholic acid uptake, whereas sodium-independent taurocholic acid uptake was unchanged. In vivo, this was manifested as a decreased serum BA clearance in all knockout mice. In a subset of mice, NTCP deficiency resulted in markedly elevated total serum BA concentrations, mainly composed of conjugated BAs. The hypercholanemic phenotype was rapidly triggered by a diet supplemented with ursodeoxycholic acid. Biliary BA output remained intact, while fecal BA excretion was reduced in hypercholanemic Slc10a1(-/-) mice, explained by increased Asbt and Ostα/β expression. These mice further showed reduced Asbt expression in the kidney and increased renal BA excretion. Hepatic uptake of conjugated BAs was potentially affected by down-regulation of OATP1A1 and up-regulation of OATP1A4. Furthermore, sodium-dependent taurocholic acid uptake was inhibited by Myrcludex B in wild-type hepatocytes, while Slc10a1(-/-) hepatocytes were insensitive to Myrcludex B. Finally, positron emission tomography showed a complete abrogation of hepatic binding of labeled Myrcludex B in Slc10a1(-/-) mice.
Conclusion: The Slc10a1-knockout mouse model supports the central role of NTCP in hepatic uptake of conjugated BAs and hepatitis B virus preS1/Myrcludex B binding in vivo; the NTCP-independent hepatic BA uptake machinery maintains a (slower) enterohepatic circulation of BAs, although it is occasionally insufficient to clear BAs from the circulation.
© 2015 by the American Association for the Study of Liver Diseases.
Figures

Comment in
-
The Na(+) -taurocholate cotransporting polypeptide knockout mouse: A new tool for study of bile acids and hepatitis B virus biology.Hepatology. 2015 Jul;62(1):19-21. doi: 10.1002/hep.27780. Epub 2015 Apr 8. Hepatology. 2015. PMID: 25761948 Free PMC article. No abstract available.
Similar articles
-
Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice.Hepatology. 2017 Nov;66(5):1631-1643. doi: 10.1002/hep.29251. Epub 2017 Sep 29. Hepatology. 2017. PMID: 28498614 Free PMC article.
-
Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes.Gastroenterology. 2014 Apr;146(4):1070-83. doi: 10.1053/j.gastro.2013.12.024. Epub 2013 Dec 19. Gastroenterology. 2014. PMID: 24361467
-
Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide.J Virol. 2014 Mar;88(6):3273-84. doi: 10.1128/JVI.03478-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390325 Free PMC article.
-
Molecular regulation of the hepatic bile acid uptake transporter and HBV entry receptor NTCP.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Aug;1866(8):158960. doi: 10.1016/j.bbalip.2021.158960. Epub 2021 Apr 29. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33932583 Review.
-
The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation.Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. Handb Exp Pharmacol. 2011. PMID: 21103971 Review.
Cited by
-
Association of the Hepatitis B Virus Large Surface Protein with Viral Infectivity and Endoplasmic Reticulum Stress-mediated Liver Carcinogenesis.Cells. 2020 Sep 8;9(9):2052. doi: 10.3390/cells9092052. Cells. 2020. PMID: 32911838 Free PMC article. Review.
-
The kinesin KIF4 mediates HBV/HDV entry through the regulation of surface NTCP localization and can be targeted by RXR agonists in vitro.PLoS Pathog. 2022 Mar 21;18(3):e1009983. doi: 10.1371/journal.ppat.1009983. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35312737 Free PMC article.
-
Primary biliary cholangitis: molecular pathogenesis perspectives and therapeutic potential of natural products.Front Immunol. 2023 Jun 30;14:1164202. doi: 10.3389/fimmu.2023.1164202. eCollection 2023. Front Immunol. 2023. PMID: 37457696 Free PMC article. Review.
-
Hepatitis Delta Virus: Replication Strategy and Upcoming Therapeutic Options for a Neglected Human Pathogen.Viruses. 2017 Jul 4;9(7):172. doi: 10.3390/v9070172. Viruses. 2017. PMID: 28677645 Free PMC article. Review.
-
The Culprit Behind HBV-Infected Hepatocytes: NTCP.Drug Des Devel Ther. 2024 Oct 28;18:4839-4858. doi: 10.2147/DDDT.S480151. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39494152 Free PMC article. Review.
References
-
- Hofmann AF. Bile acids: the good, the bad, and the ugly. News Physiol Sci. 1999;14:24–29. - PubMed
-
- Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. - PubMed
-
- Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
