Implication of delta opioid receptor subtype 2 but not delta opioid receptor subtype 1 in the development of morphine analgesic tolerance in a rat model of chronic inflammatory pain
- PMID: 25639561
- PMCID: PMC4390435
- DOI: 10.1111/ejn.12829
Implication of delta opioid receptor subtype 2 but not delta opioid receptor subtype 1 in the development of morphine analgesic tolerance in a rat model of chronic inflammatory pain
Abstract
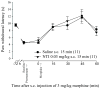
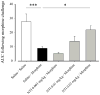
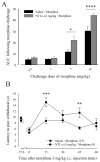
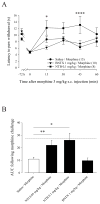
Opioids are well known for their robust analgesic effects. Chronic activation of mu opioid receptors (MOPs) is, however, accompanied by various unwanted effects such as analgesic tolerance. Among other mechanisms, interactions between MOPs and delta opioid receptors (DOPs) are thought to play an important role in morphine-induced behavioral adaptations. Interestingly, certain conditions such as inflammation enhance the function of the DOP through a MOP-dependent mechanism. Here, we investigated the role of DOPs during the development of morphine tolerance in an animal model of chronic inflammatory pain. Using behavioral approaches, we first established that repeated systemic morphine treatment induced morphine analgesic tolerance in rats coping with chronic inflammatory pain. We then observed that blockade of DOPs with subcutaneous naltrindole (NTI), a selective DOP antagonist, significantly attenuated the development of morphine tolerance in a dose-dependent manner. We confirmed that this effect was DOP mediated by showing that an acute injection of NTI had no effect on morphine-induced analgesia in naive animals. Previous pharmacological characterizations revealed the existence of DOP subtype 1 and DOP subtype 2. As opposed to NTI, 7-benzylidenenaltrexone and naltriben were reported to be selective DOP subtype 1 and DOP subtype 2 antagonists, respectively. Interestingly, naltriben but not 7-benzylidenenaltrexone was able to attenuate the development of morphine analgesic tolerance in inflamed rats. Altogether, our results suggest that targeting of DOP subtype 2 with antagonists provides a valuable strategy to attenuate the analgesic tolerance that develops after repeated morphine administration in the setting of chronic inflammatory pain.
Keywords: antagonist; heteromer; hyperalgesia; inflammation; opioid.
© 2015 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures

Similar articles
-
Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists.Br J Pharmacol. 2007 Jul;151(6):877-87. doi: 10.1038/sj.bjp.0707277. Epub 2007 May 14. Br J Pharmacol. 2007. PMID: 17502848 Free PMC article.
-
Delta(2)-opioid receptor mediation of morphine-induced CCK release in the frontal cortex of the freely moving rat.Synapse. 1999 Oct;34(1):47-54. doi: 10.1002/(SICI)1098-2396(199910)34:1<47::AID-SYN6>3.0.CO;2-9. Synapse. 1999. PMID: 10459171
-
Inverse agonist action of Leu-enkephalin at delta(2)-opioid receptors mediates spinal antianalgesia.J Pharmacol Exp Ther. 2001 May;297(2):582-9. J Pharmacol Exp Ther. 2001. PMID: 11303046
-
Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance.Trends Pharmacol Sci. 2006 Jun;27(6):324-9. doi: 10.1016/j.tips.2006.04.005. Epub 2006 May 6. Trends Pharmacol Sci. 2006. PMID: 16678916 Review.
-
Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile.Br J Anaesth. 2009 Jul;103(1):38-49. doi: 10.1093/bja/aep129. Epub 2009 May 27. Br J Anaesth. 2009. PMID: 19474215 Review.
Cited by
-
Neuropeptide and cytokine regulation of pain in the context of substance use disorders.Neuropharmacology. 2020 Sep 1;174:108153. doi: 10.1016/j.neuropharm.2020.108153. Epub 2020 May 26. Neuropharmacology. 2020. PMID: 32470337 Free PMC article. Review.
-
Replication-Competent Influenza Virus and Respiratory Syncytial Virus Luciferase Reporter Strains Engineered for Co-Infections Identify Antiviral Compounds in Combination Screens.Biochemistry. 2015 Sep 15;54(36):5589-604. doi: 10.1021/acs.biochem.5b00623. Epub 2015 Sep 1. Biochemistry. 2015. PMID: 26307636 Free PMC article.
-
Endogenous opioid systems alterations in pain and opioid use disorder.Front Syst Neurosci. 2022 Oct 19;16:1014768. doi: 10.3389/fnsys.2022.1014768. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36341476 Free PMC article. Review.
-
Molecular Pharmacology of δ-Opioid Receptors.Pharmacol Rev. 2016 Jul;68(3):631-700. doi: 10.1124/pr.114.008979. Pharmacol Rev. 2016. PMID: 27343248 Free PMC article. Review.
-
Placental microRNA methylome signatures may serve as biomarkers and therapeutic targets for prenatally opioid-exposed infants with neonatal opioid withdrawal syndrome.Front Genet. 2023 Jun 15;14:1215472. doi: 10.3389/fgene.2023.1215472. eCollection 2023. Front Genet. 2023. PMID: 37434949 Free PMC article.
References
-
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. - PubMed
-
- Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47:2969–2972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

