Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human
- PMID: 25637376
- PMCID: PMC4368892
- DOI: 10.1093/ijnp/pyu036
Determining pharmacological selectivity of the kappa opioid receptor antagonist LY2456302 using pupillometry as a translational biomarker in rat and human
Abstract
Background: Selective kappa opioid receptor antagonism is a promising experimental strategy for the treatment of depression. The kappa opioid receptor antagonist, LY2456302, exhibits ~30-fold higher affinity for kappa opioid receptors over mu opioid receptors, which is the next closest identified pharmacology.
Methods: Here, we determined kappa opioid receptor pharmacological selectivity of LY2456302 by assessing mu opioid receptor antagonism using translational pupillometry in rats and humans.
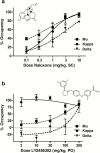
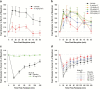
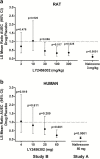
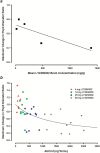
Results: In rats, morphine-induced mydriasis was completely blocked by the nonselective opioid receptor antagonist naloxone (3mg/kg, which produced 90% mu opioid receptor occupancy), while 100 and 300 mg/kg LY2456302 (which produced 56% and 87% mu opioid receptor occupancy, respectively) only partially blocked morphine-induced mydriasis. In humans, fentanyl-induced miosis was completely blocked by 50mg naltrexone, and LY2456302 dose-dependently blocked miosis at 25 and 60 mg (minimal-to-no blockade at 4-10mg).
Conclusions: We demonstrate, for the first time, the use of translational pupillometry in the context of receptor occupancy to identify a clinical dose of LY2456302 achieving maximal kappa opioid receptor occupancy without evidence of significant mu receptor antagonism.
Keywords: LY2456302; dynorphin; kappa opioid receptor; pupillometry; translational biomarker.
© The Author 2015. Published by Oxford University Press on behalf of CINP.
Figures
Similar articles
-
LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders.Neuropharmacology. 2014 Feb;77:131-44. doi: 10.1016/j.neuropharm.2013.09.021. Epub 2013 Sep 23. Neuropharmacology. 2014. PMID: 24071566
-
Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects.J Clin Pharmacol. 2014 Sep;54(9):968-78. doi: 10.1002/jcph.286. Epub 2014 Mar 26. J Clin Pharmacol. 2014. PMID: 24619932 Clinical Trial.
-
Kappa opioid antagonist effects of the novel kappa antagonist 5'-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys.Psychopharmacology (Berl). 2002 Oct;163(3-4):412-9. doi: 10.1007/s00213-002-1038-x. Epub 2002 Mar 13. Psychopharmacology (Berl). 2002. PMID: 12373442
-
The Role of Dynorphin and the Kappa Opioid Receptor in the Symptomatology of Schizophrenia: A Review of the Evidence.Biol Psychiatry. 2019 Oct 1;86(7):502-511. doi: 10.1016/j.biopsych.2019.05.012. Epub 2019 May 22. Biol Psychiatry. 2019. PMID: 31376930 Review.
-
Preclinical and clinical efficacy of kappa opioid receptor antagonists for depression: A systematic review.J Affect Disord. 2024 Oct 1;362:816-827. doi: 10.1016/j.jad.2024.07.030. Epub 2024 Jul 15. J Affect Disord. 2024. PMID: 39019223 Review.
Cited by
-
Impact of Pharmacological Manipulation of the κ-Opioid Receptor System on Self-grooming and Anhedonic-like Behaviors in Male Mice.J Pharmacol Exp Ther. 2019 Jul;370(1):1-8. doi: 10.1124/jpet.119.256354. Epub 2019 Apr 11. J Pharmacol Exp Ther. 2019. PMID: 30975792 Free PMC article.
-
Sex differences in kappa opioid receptor inhibition of latent postoperative pain sensitization in dorsal horn.Neuropharmacology. 2020 Feb;163:107726. doi: 10.1016/j.neuropharm.2019.107726. Epub 2019 Jul 25. Neuropharmacology. 2020. PMID: 31351975 Free PMC article.
-
A randomized, double-blind, placebo-controlled study of the kappa opioid receptor antagonist, CERC-501, in a human laboratory model of smoking behavior.Addict Biol. 2020 Jul;25(4):e12799. doi: 10.1111/adb.12799. Epub 2019 Jun 26. Addict Biol. 2020. PMID: 31240842 Free PMC article.
-
Targeting opioid dysregulation in depression for the development of novel therapeutics.Pharmacol Ther. 2019 Sep;201:51-76. doi: 10.1016/j.pharmthera.2019.04.009. Epub 2019 Apr 30. Pharmacol Ther. 2019. PMID: 31051197 Free PMC article. Review.
-
In vivo Characterization of the Opioid Receptor-Binding Profiles of Samidorphan and Naltrexone in Rats: Comparisons at Clinically Relevant Concentrations.Neuropsychiatr Dis Treat. 2022 Nov 1;18:2497-2506. doi: 10.2147/NDT.S373195. eCollection 2022. Neuropsychiatr Dis Treat. 2022. PMID: 36345421 Free PMC article.
References
-
- Berton O, Nestler EJ. (2006). New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151. - PubMed
-
- Diaz-Buezo N, Pedregal-Tercero C, McKinzie D, Mitch C. (2009). Kappa selective opioid receptor antagonist. In: US Patent # US20090186873. Issued to Eli Lilly and Company; USA.
-
- Greenwald MK, June HL, Stitzer ML, Marco AP. (1996). Comparative clinical pharmacology of short-acting mu opioids in drug abusers. J Pharmacol Exp Ther 277:1228–1236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

