Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression
- PMID: 25635942
- PMCID: PMC5414753
- DOI: 10.1210/me.2014-1390
Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression
Abstract
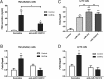
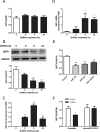
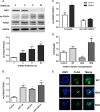
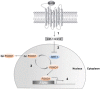
GnRH plays a key role in the vertebrate reproductive system by stimulating biosynthesis and secretion of pituitary gonadotropins. However, the potential involvement of microRNAs (miRNAs) on this activation has still to be explored. In this study, we investigated the role of miRNA-132 and miRNA-212, two tandemly expressed miRNAs that target the same transcripts, on GnRH-induced FSH expression. We first showed that the GnRH stimulation of FSH secretion was reduced and Fshb mRNA abolished by blocking miR-132/212 action in rat pituitary cells. In mouse LβT2 gonadotrope cells, the GnRH stimulation of Fshb mRNA was also demonstrated to be dependent on miR-132/212 and reproduced by overexpressing one or both miRNAs. We then showed that the miR-132/212-mediated action of GnRH involved a posttranscriptional decrease of sirtuin 1 (SIRT1) deacetylase. The lower level of SIRT1 deacetylase correlated with an increase in the acetylated form of Forkhead Box O1 (FOXO1), a transcriptional repressor of Fshb. Interestingly, we show that the acetylated mimicking mutant of FOXO1 was localized outside the nucleus, thus alleviating its repressive effect on Fshb transcription. Overall, we demonstrate that the GnRH stimulation of Fshb expression is dependent on miR-132/212 and involves a SIRT1-FOXO1 pathway. This is the first demonstration of an obligatory microRNA pathway in the GnRH-regulated expression of a gonadotropin gene.
Figures
Similar articles
-
Forkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropes.Mol Endocrinol. 2013 Nov;27(11):1825-39. doi: 10.1210/me.2013-1185. Epub 2013 Sep 24. Mol Endocrinol. 2013. PMID: 24065703 Free PMC article.
-
GnRH Pulse Frequency Control of Fshb Gene Expression Is Mediated via ERK1/2 Regulation of ICER.Mol Endocrinol. 2016 Mar;30(3):348-60. doi: 10.1210/me.2015-1222. Epub 2016 Feb 2. Mol Endocrinol. 2016. PMID: 26835742 Free PMC article.
-
Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1.Mol Endocrinol. 2011 Aug;25(8):1387-403. doi: 10.1210/me.2011-0032. Epub 2011 Jun 9. Mol Endocrinol. 2011. PMID: 21659477 Free PMC article.
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
-
The nature of FSH induction by GnRH.Trends Endocrinol Metab. 2002 Aug;13(6):257-63. doi: 10.1016/s1043-2760(02)00614-8. Trends Endocrinol Metab. 2002. PMID: 12128287 Review.
Cited by
-
Pituitary tissue-specific miR-7a-5p regulates FSH expression in rat anterior adenohypophyseal cells.PeerJ. 2019 Apr 9;7:e6458. doi: 10.7717/peerj.6458. eCollection 2019. PeerJ. 2019. PMID: 30993031 Free PMC article.
-
circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells.Nucleic Acids Res. 2019 Apr 23;47(7):3580-3593. doi: 10.1093/nar/gkz141. Nucleic Acids Res. 2019. PMID: 30820544 Free PMC article.
-
Editorial: MicroRNAs in endocrinology and cell signaling.Front Endocrinol (Lausanne). 2022 Dec 19;13:1118426. doi: 10.3389/fendo.2022.1118426. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36601018 Free PMC article. No abstract available.
-
Reactive Oxygen Species Link Gonadotropin-Releasing Hormone Receptor Signaling Cascades in the Gonadotrope.Front Endocrinol (Lausanne). 2017 Oct 30;8:286. doi: 10.3389/fendo.2017.00286. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29163358 Free PMC article.
-
Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction.Int J Environ Res Public Health. 2021 Jan 30;18(3):1243. doi: 10.3390/ijerph18031243. Int J Environ Res Public Health. 2021. PMID: 33573212 Free PMC article. Review.
References
-
- Counis R, Laverriere JN, Garrel G, et al. . Gonadotropin-releasing hormone and the control of gonadotrope function. Reprod Nutr Dev. 2005;45:243–254. - PubMed
-
- Huhtaniemi I. Mutations along the pituitary-gonadal axis affecting sexual maturation: novel information from transgenic and knockout mice. Mol Cell Endocrinol. 2006;254–255:84–90. - PubMed
-
- Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSH β subunit gene is highly conserved. Mol Hum Reprod. 2005;11:601–605. - PubMed
-
- Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

