Epidemiology, ecology and gene pool of influenza A virus in Egypt: will Egypt be the epicentre of the next influenza pandemic?
- PMID: 25635701
- PMCID: PMC4603438
- DOI: 10.4161/21505594.2014.992662
Epidemiology, ecology and gene pool of influenza A virus in Egypt: will Egypt be the epicentre of the next influenza pandemic?
Abstract
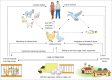
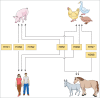
Outside Asia, Egypt is considered to be an influenza H5N1 epicentre and presents a far greater pandemic risk than other countries. The long-term endemicity of H5N1 and the recent emergence of H9N2 in poultry call attention to the need for unravelling the epidemiology, ecology and highly diverse gene pool of influenza A virus (IAV) in Egypt which is the aim of this review. Isolation of a considerable number of IAV subtypes from several avian and mammalian hosts was described. Co-infections of poultry with H5N1 and H9N2 and subclinical infections of pigs and humans with H1N1 and H5N1 may raise the potential for the reassortment of these viruses. Moreover, the adjustment of IAV genomes, particularly H5N1, to optimize their evolution toward efficient transmission in human is progressing in Egypt. Understanding the present situation of influenza viruses in Egypt will help in the control of the disease and can potentially prevent a possible pandemic.
Keywords: ELISA, Enzyme linked immunosorbent assay; Egypt; H5N1; H9N2; HA, hemagglutinin; HI, hemagglutination inhibition test; HPAIV, highly pathogenic avian influenza viruses; IAV, influenza A viruses; LBM, live bird markets; LPAIV, low pathogenic avian influenza viruses; M, matrix; NA, neuraminidase; NAMRU-3, Naval Medical Research Unit–3; NLQP, National Laboratory for Veterinary Quality Control on Poultry Production; NS, non-structural; PA, acidic polymerase; PB, basic polymerase; WHO, World Health Organization; epidemiology; influenza; pandemic; reassortment; virulence.
Figures
Comment in
-
Pandemic influenza virus: tracking a three-headed monster.Virulence. 2015;6(5):405-6. doi: 10.1080/21505594.2015.1020275. Epub 2015 Mar 4. Virulence. 2015. PMID: 25738965 Free PMC article. No abstract available.
Similar articles
-
Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt.Viruses. 2018 Mar 9;10(3):121. doi: 10.3390/v10030121. Viruses. 2018. PMID: 29522492 Free PMC article. Review.
-
Evolutionary trajectories and diagnostic challenges of potentially zoonotic avian influenza viruses H5N1 and H9N2 co-circulating in Egypt.Infect Genet Evol. 2015 Aug;34:278-91. doi: 10.1016/j.meegid.2015.06.004. Epub 2015 Jun 3. Infect Genet Evol. 2015. PMID: 26049044
-
Natural Reassortants of Potentially Zoonotic Avian Influenza Viruses H5N1 and H9N2 from Egypt Display Distinct Pathogenic Phenotypes in Experimentally Infected Chickens and Ferrets.J Virol. 2017 Nov 14;91(23):e01300-17. doi: 10.1128/JVI.01300-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931674 Free PMC article.
-
Evidence of infection with avian, human, and swine influenza viruses in pigs in Cairo, Egypt.Arch Virol. 2018 Feb;163(2):359-364. doi: 10.1007/s00705-017-3619-3. Epub 2017 Oct 26. Arch Virol. 2018. PMID: 29075888
-
(Highly pathogenic) avian influenza as a zoonotic agent.Vet Microbiol. 2010 Jan 27;140(3-4):237-45. doi: 10.1016/j.vetmic.2009.08.022. Epub 2009 Aug 26. Vet Microbiol. 2010. PMID: 19782482 Review.
Cited by
-
Serological and Molecular Identification of Brucella spp. in Pigs from Cairo and Giza Governorates, Egypt.Pathogens. 2019 Nov 20;8(4):248. doi: 10.3390/pathogens8040248. Pathogens. 2019. PMID: 31756893 Free PMC article.
-
An Overview of the Most Significant Zoonotic Viral Pathogens Transmitted from Animal to Human in Saudi Arabia.Pathogens. 2019 Feb 22;8(1):25. doi: 10.3390/pathogens8010025. Pathogens. 2019. PMID: 30813309 Free PMC article. Review.
-
Challenge for One Health: Co-Circulation of Zoonotic H5N1 and H9N2 Avian Influenza Viruses in Egypt.Viruses. 2018 Mar 9;10(3):121. doi: 10.3390/v10030121. Viruses. 2018. PMID: 29522492 Free PMC article. Review.
-
Novel reassortant H9N2 viruses in pigeons and evidence for antigenic diversity of H9N2 viruses isolated from quails in Egypt.J Gen Virol. 2017 Apr;98(4):548-562. doi: 10.1099/jgv.0.000657. Epub 2017 May 5. J Gen Virol. 2017. PMID: 27902350 Free PMC article.
-
A Comprehensive Review of Common Bacterial, Parasitic and Viral Zoonoses at the Human-Animal Interface in Egypt.Pathogens. 2017 Jul 21;6(3):33. doi: 10.3390/pathogens6030033. Pathogens. 2017. PMID: 28754024 Free PMC article. Review.
References
-
- Yee KS, Carpenter TE, Cardona CJ. Epidemiology of H5N1 avian influenza. Comp Immunol Microbiol Infect Dis 2009; 32:325-40; PMID:18448168; http://dx.doi.org/10.1016/j.cimid.2008.01.005 - DOI - PubMed
-
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25:5637-44; PMID:17126960; http://dx.doi.org/10.1016/j.vaccine.2006.10.051 - DOI - PubMed
-
- Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, Recuenco S, Ellison JA, Davis CT, York IA, et al. . A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A 2012; 109:4269-74; PMID:22371588; http://dx.doi.org/10.1073/pnas.1116200109 - DOI - PMC - PubMed
-
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, et al. . New world bats harbor diverse influenza a viruses. PLoS Pathog 2013; 9:e1003657; PMID:24130481; http://dx.doi.org/10.1371/journal.ppat.1003657 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
