The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration
- PMID: 25631089
- PMCID: PMC4442405
- DOI: 10.1128/JVI.03574-14
The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration
Abstract
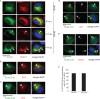
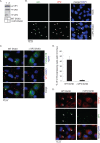
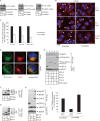
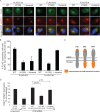
The nonenveloped simian virus 40 (SV40) hijacks the three endoplasmic reticulum (ER) membrane-bound J proteins B12, B14, and C18 to escape from the ER into the cytosol en route to successful infection. How C18 controls SV40 ER-to-cytosol membrane penetration is the least understood of these processes. We previously found that SV40 triggers B12 and B14 to reorganize into discrete puncta in the ER membrane called foci, structures postulated to represent the cytosol entry site (C. P. Walczak, M. S. Ravindran, T. Inoue, and B. Tsai, PLoS Pathog 10: e1004007, 2014). We now find that SV40 also recruits C18 to the virus-induced B12/B14 foci. Importantly, the C18 foci harbor membrane penetration-competent SV40, further implicating this structure as the membrane penetration site. Consistent with this, a mutant SV40 that cannot penetrate the ER membrane and promote infection fails to induce C18 foci. C18 also regulates the recruitment of B12/B14 into the foci. In contrast to B14, C18's cytosolic Hsc70-binding J domain, but not the lumenal domain, is essential for its targeting to the foci; this J domain likewise is necessary to support SV40 infection. Knockdown-rescue experiments reveal that C18 executes a role that is not redundant with those of B12/B14 during SV40 infection. Collectively, our data illuminate C18's contribution to SV40 ER membrane penetration, strengthening the idea that SV40-triggered foci are critical for cytosol entry.
Importance: Polyomaviruses (PyVs) cause devastating human diseases, particularly in immunocompromised patients. As this virus family continues to be a significant human pathogen, clarifying the molecular basis of their cellular entry pathway remains a high priority. To infect cells, PyV traffics from the cell surface to the ER, where it penetrates the ER membrane to reach the cytosol. In the cytosol, the virus moves to the nucleus to cause infection. ER-to-cytosol membrane penetration is a critical yet mysterious infection step. In this study, we clarify the role of an ER membrane protein called C18 in mobilizing the simian PyV SV40, a PyV archetype, from the ER into the cytosol. Our findings also support the hypothesis that SV40 induces the formation of punctate structures in the ER membrane, called foci, that serve as the portal for cytosol entry of the virus.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
How Polyomaviruses Exploit the ERAD Machinery to Cause Infection.Viruses. 2016 Aug 29;8(9):242. doi: 10.3390/v8090242. Viruses. 2016. PMID: 27589785 Free PMC article. Review.
-
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review.
-
A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40.J Virol. 2015 Apr;89(8):4069-79. doi: 10.1128/JVI.03552-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653441 Free PMC article.
-
SGTA-Dependent Regulation of Hsc70 Promotes Cytosol Entry of Simian Virus 40 from the Endoplasmic Reticulum.J Virol. 2017 May 26;91(12):e00232-17. doi: 10.1128/JVI.00232-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28356524 Free PMC article.
-
ERdj5 Reductase Cooperates with Protein Disulfide Isomerase To Promote Simian Virus 40 Endoplasmic Reticulum Membrane Translocation.J Virol. 2015 Sep;89(17):8897-908. doi: 10.1128/JVI.00941-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085143 Free PMC article.
Cited by
-
A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane.PLoS Pathog. 2015 Aug 5;11(8):e1005086. doi: 10.1371/journal.ppat.1005086. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26244546 Free PMC article.
-
How Polyomaviruses Exploit the ERAD Machinery to Cause Infection.Viruses. 2016 Aug 29;8(9):242. doi: 10.3390/v8090242. Viruses. 2016. PMID: 27589785 Free PMC article. Review.
-
Components of the LINC and NPC complexes coordinately target and translocate a virus into the nucleus to promote infection.PLoS Pathog. 2022 Sep 6;18(9):e1010824. doi: 10.1371/journal.ppat.1010824. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36067270 Free PMC article.
-
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review.
-
Principles of Virus Uncoating: Cues and the Snooker Ball.Traffic. 2016 Jun;17(6):569-92. doi: 10.1111/tra.12387. Epub 2016 Mar 31. Traffic. 2016. PMID: 26875443 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

