Bone-remodeling transcript levels are independent of perching in end-of-lay white leghorn chickens
- PMID: 25625518
- PMCID: PMC4346857
- DOI: 10.3390/ijms16022663
Bone-remodeling transcript levels are independent of perching in end-of-lay white leghorn chickens
Abstract
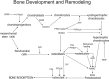
Osteoporosis is a bone disease that commonly results in a 30% incidence of fracture in hens used to produce eggs for human consumption. One of the causes of osteoporosis is the lack of mechanical strain placed on weight-bearing bones. In conventionally-caged hens, there is inadequate space for chickens to exercise and induce mechanical strain on their bones. One approach is to encourage mechanical stress on bones by the addition of perches to conventional cages. Our study focuses on the molecular mechanism of bone remodeling in end-of-lay hens (71 weeks) with access to perches. We examined bone-specific transcripts that are actively involved during development and remodeling. Using real-time quantitative PCR, we examined seven transcripts (COL2A1 (collagen, type II, alpha 1), RANKL (receptor activator of nuclear factor kappa-B ligand), OPG (osteoprotegerin), PTHLH (PTH-like hormone), PTH1R (PTH/PTHLH type-1 receptor), PTH3R (PTH/PTHLH type-3 receptor), and SOX9 (Sry-related high mobility group box)) in phalange, tibia and femur. Our results indicate that the only significant effect was a difference among bones for COL2A1 (femur > phalange). Therefore, we conclude that access to a perch did not alter transcript expression. Furthermore, because hens have been used as a model for human bone metabolism and osteoporosis, the results indicate that bone remodeling due to mechanical loading in chickens may be a product of different pathways than those involved in the mammalian model.
Figures
Similar articles
-
Serum PTH, PTH1R/ATF4 pathway, and the sRANKL/OPG system in bone as a new link between bone growth, cross-sectional geometry, and strength in young rats with experimental chronic kidney disease.Cytokine. 2021 Dec;148:155685. doi: 10.1016/j.cyto.2021.155685. Epub 2021 Aug 16. Cytokine. 2021. PMID: 34411988
-
RANKL/OPG system regulation by endogenous PTH and PTH1R/ATF4 axis in bone: Implications for bone accrual and strength in growing rats with mild uremia.Cytokine. 2018 Jun;106:19-28. doi: 10.1016/j.cyto.2018.03.002. Epub 2018 Mar 9. Cytokine. 2018. PMID: 29529595
-
Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells.J Bone Miner Res. 2002 Sep;17(9):1667-79. doi: 10.1359/jbmr.2002.17.9.1667. J Bone Miner Res. 2002. PMID: 12211438
-
[Role of OPG in regulation of bone remodeling].Clin Calcium. 2006 Sep;16(9):1463-68. Clin Calcium. 2006. PMID: 16951469 Review. Japanese.
-
RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives.J Exp Clin Cancer Res. 2019 Jan 8;38(1):12. doi: 10.1186/s13046-018-1001-2. J Exp Clin Cancer Res. 2019. PMID: 30621730 Free PMC article. Review.
Cited by
-
Effects of rearing systems on the eggshell quality, bone parameters and expression of genes related to bone remodeling in aged laying hens.Front Physiol. 2022 Aug 31;13:962330. doi: 10.3389/fphys.2022.962330. eCollection 2022. Front Physiol. 2022. PMID: 36117717 Free PMC article.
-
Identifying artificial selection signals in the chicken genome.PLoS One. 2018 Apr 26;13(4):e0196215. doi: 10.1371/journal.pone.0196215. eCollection 2018. PLoS One. 2018. PMID: 29698423 Free PMC article.
-
Cortistatin prevents glucocorticoid-associated osteonecrosis of the femoral head via the GHSR1a/Akt pathway.Commun Biol. 2024 Jan 26;7(1):132. doi: 10.1038/s42003-024-05795-5. Commun Biol. 2024. PMID: 38278996 Free PMC article.
-
Dietary Soybean Oil Supplementation Affects Keel Bone Characters and Daily Feed Intake but Not Egg Production and Quality in Laying Hens Housed in Furnished Cages.Front Vet Sci. 2021 Mar 19;8:657585. doi: 10.3389/fvets.2021.657585. eCollection 2021. Front Vet Sci. 2021. PMID: 33816591 Free PMC article.
References
-
- Saini V., Marengi D., Barry K., Fulzele K., Heiden E., Liu X., Dedic C., Maeda A., Lotinun S., Baron R., et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 reeptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 2013;288:20122–20134. doi: 10.1074/jbc.M112.441360. - DOI - PMC - PubMed
-
- Dacke C., Arkle S., Cook D., Wormstone I., Jones S., Zaidi M., Bascal Z. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993;184:63–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

