Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at β2*nicotinic acetylcholine receptors
- PMID: 25625469
- PMCID: PMC4439881
- DOI: 10.1111/bph.13090
Anxiolytic-like and anxiogenic-like effects of nicotine are regulated via diverse action at β2*nicotinic acetylcholine receptors
Abstract
Background and purpose: Nicotine dose-dependently activates or preferentially desensitizes β2 subunit containing nicotinic ACh receptors (β2*nAChRs). Genetic and pharmacological manipulations assessed effects of stimulation versus inhibition of β2*nAChRs on nicotine-associated anxiety-like phenotype.
Experimental approach: Using a range of doses of nicotine in β2*nAChR subunit null mutant mice (β2KO; backcrossed to C57BL/6J) and their wild-type (WT) littermates, administration of the selective β2*nAChR agonist, 5I-A85380, and the selective β2*nAChR antagonist dihydro-β-erythroidine (DHβE), we determined the behavioural effects of stimulation and inhibition of β2*nAChRs in the light-dark and elevated plus maze (EPM) assays.
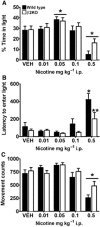
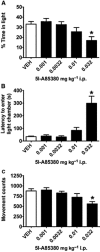
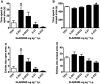
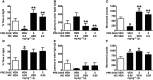
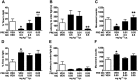
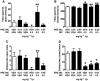
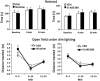
Key results: Low-dose i.p. nicotine (0.05 mg·kg(-) 1) supported anxiolysis-like behaviour independent of genotype whereas the highest dose (0.5 mg·kg(-1) ) promoted anxiogenic-like phenotype in WT mice, but was blunted in β2KO mice for the measure of latency. Administration of 5I-A85380 had similar dose-dependent effects in C57BL/6J WT mice; 0.001 mg·kg(-1) 5I-A85380 reduced anxiety on an EPM, whereas 0.032 mg·kg(-1) 5I-A85380 promoted anxiogenic-like behaviour in both the light-dark and EPM assays. DHβE pretreatment blocked anxiogenic-like effects of 0.5 mg·kg(-1) nicotine. Similarly to DHβE, pretreatment with low-dose 0.05 mg·kg(-1) nicotine did not accumulate with 0.5 mg·kg(-1) nicotine, but rather blocked anxiogenic-like effects of high-dose nicotine in the light-dark and EPM assays.
Conclusions and implications: These studies provide direct evidence that low-dose nicotine inhibits nAChRs and demonstrate that inhibition or stimulation of β2*nAChRs supports the corresponding anxiolytic-like or anxiogenic-like effects of nicotine. Inhibition of β2*nAChRs may relieve anxiety in smokers and non-smokers alike.
© 2015 The British Pharmacological Society.
Figures
Similar articles
-
Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice.PLoS One. 2012;7(11):e48665. doi: 10.1371/journal.pone.0048665. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144922 Free PMC article.
-
Do different mechanisms underlie two anxiogenic effects of systemic nicotine?Behav Pharmacol. 2003 Jul;14(4):323-9. doi: 10.1097/01.fbp.0000081782.35927.c6. Behav Pharmacol. 2003. PMID: 12838038
-
A role for α4(non-α6)* nicotinic acetylcholine receptors in motor behavior.Neuropharmacology. 2013 Oct;73:19-30. doi: 10.1016/j.neuropharm.2013.05.001. Epub 2013 May 17. Neuropharmacology. 2013. PMID: 23688922 Free PMC article.
-
Desensitization of nicotinic acetylcholine receptors as a strategy for drug development.J Pharmacol Exp Ther. 2009 Feb;328(2):364-70. doi: 10.1124/jpet.108.145292. Epub 2008 Nov 20. J Pharmacol Exp Ther. 2009. PMID: 19023041 Free PMC article. Review.
-
Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications.Pharmacol Ther. 2001 Nov-Dec;92(2-3):89-108. doi: 10.1016/s0163-7258(01)00161-9. Pharmacol Ther. 2001. PMID: 11916531 Review.
Cited by
-
Substance Use, Financial Stress, Employment Disruptions, and Anxiety among Veterans during the COVID-19 Pandemic.Psychol Rep. 2023 Aug;126(4):1684-1700. doi: 10.1177/00332941221080413. Epub 2022 Mar 24. Psychol Rep. 2023. PMID: 35324356 Free PMC article.
-
Nicotine-free vapor inhalation produces behavioral disruptions and anxiety-like behaviors in mice: Effects of puff duration, session length, sex, and flavor.Pharmacol Biochem Behav. 2021 Jul;206:173207. doi: 10.1016/j.pbb.2021.173207. Epub 2021 May 19. Pharmacol Biochem Behav. 2021. PMID: 34019915 Free PMC article.
-
In utero and Lactational Exposure to Acetamiprid Induces Abnormalities in Socio-Sexual and Anxiety-Related Behaviors of Male Mice.Front Neurosci. 2016 Jun 3;10:228. doi: 10.3389/fnins.2016.00228. eCollection 2016. Front Neurosci. 2016. PMID: 27375407 Free PMC article.
-
Chronic nicotine impairs sparse motor learning via striatal fast-spiking parvalbumin interneurons.Addict Biol. 2021 May;26(3):e12956. doi: 10.1111/adb.12956. Epub 2020 Aug 6. Addict Biol. 2021. PMID: 32767546 Free PMC article.
-
C57BL/6 Substrain Differences in Pharmacological Effects after Acute and Repeated Nicotine Administration.Brain Sci. 2019 Sep 21;9(10):244. doi: 10.3390/brainsci9100244. Brain Sci. 2019. PMID: 31546627 Free PMC article.
References
-
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. - PubMed
-
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

