Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions
- PMID: 25624468
- PMCID: PMC4330750
- DOI: 10.1073/pnas.1416276112
Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions
Abstract
The generation and functions of human peripheral blood (PB) IgM(+)IgD(+)CD27(+) B lymphocytes with somatically mutated IgV genes are controversially discussed. We determined their differential gene expression to naive B cells and to IgM-only and IgG(+) memory B cells. This analysis revealed a high similarity of IgM(+)(IgD(+))CD27(+) and IgG(+) memory B cells but also pointed at distinct functional capacities of both subsets. In vitro analyses revealed a tendency of activated IgM(+)IgD(+)CD27(+) B cells to migrate to B-cell follicles and undergo germinal center (GC) B-cell differentiation, whereas activated IgG(+) memory B cells preferentially showed a plasma cell (PC) fate. This observation was supported by reverse regulation of B-cell lymphoma 6 and PR domain containing 1 and differential BTB and CNC homology 1, basic leucine zipper transcription factor 2 expression. Moreover, IgM(+)IgD(+)CD27(+) B lymphocytes preferentially responded to neutrophil-derived cytokines. Costimulation with catecholamines, carcinoembryonic antigen cell adhesion molecule 8 (CEACAM8), and IFN-γ caused differentiation of IgM(+)IgD(+)CD27(+) B cells into PCs, induced class switching to IgG2, and was reproducible in cocultures with neutrophils. In conclusion, this study substantiates memory B-cell characteristics of human IgM(+)IgD(+)CD27(+) B cells in that they share typical memory B-cell transcription patterns with IgG(+) post-GC B cells and show a faster and more vigorous restimulation potential, a hallmark of immune memory. Moreover, this work reveals a functional plasticity of human IgM memory B cells by showing their propensity to undergo secondary GC reactions upon reactivation, but also by their special role in early inflammation via interaction with immunomodulatory neutrophils.
Keywords: IgM memory B-cell functions; early inflammatory response; germinal center reentry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

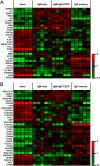
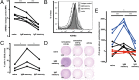



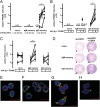
Comment in
-
B cell memory: Making sense in humans.Nat Rev Immunol. 2015 Mar;15(3):133. doi: 10.1038/nri3822. Epub 2015 Feb 6. Nat Rev Immunol. 2015. PMID: 25656708 No abstract available.
Similar articles
-
Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation.J Exp Med. 2009 Nov 23;206(12):2659-69. doi: 10.1084/jem.20091087. Epub 2009 Nov 16. J Exp Med. 2009. PMID: 19917772 Free PMC article.
-
Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells.J Exp Med. 1998 Nov 2;188(9):1679-89. doi: 10.1084/jem.188.9.1679. J Exp Med. 1998. PMID: 9802980 Free PMC article.
-
IgG subclass switch capacity is low in switched and in IgM-only, but high in IgD+IgM+, post-germinal center (CD27+) human B cells.Eur J Immunol. 2001 Jan;31(1):243-9. doi: 10.1002/1521-4141(200101)31:1<243::AID-IMMU243>3.0.CO;2-0. Eur J Immunol. 2001. PMID: 11265640
-
[IgM+IgD+CD27+ B cells in human: an essential role in the protection against encapsulated bacteria].Med Sci (Paris). 2015 Jun-Jul;31(6-7):647-53. doi: 10.1051/medsci/20153106018. Epub 2015 Jul 7. Med Sci (Paris). 2015. PMID: 26152169 Review. French.
-
Human IgM-expressing memory B cells.Front Immunol. 2023 Dec 8;14:1308378. doi: 10.3389/fimmu.2023.1308378. eCollection 2023. Front Immunol. 2023. PMID: 38143767 Free PMC article. Review.
Cited by
-
Lineage tracing reveals fate bias and transcriptional memory in human B cells.Life Sci Alliance. 2023 Jan 13;6(3):e202201792. doi: 10.26508/lsa.202201792. Print 2023 Mar. Life Sci Alliance. 2023. PMID: 36639222 Free PMC article.
-
Class-Switch Recombination Occurs Infrequently in Germinal Centers.Immunity. 2019 Aug 20;51(2):337-350.e7. doi: 10.1016/j.immuni.2019.07.001. Epub 2019 Jul 30. Immunity. 2019. PMID: 31375460 Free PMC article.
-
Instructing durable humoral immunity for COVID-19 and other vaccinable diseases.Immunity. 2022 Jun 14;55(6):945-964. doi: 10.1016/j.immuni.2022.05.004. Epub 2022 May 10. Immunity. 2022. PMID: 35637104 Free PMC article. Review.
-
Characterising the Phenotypic Diversity of Antigen-Specific Memory B Cells Before and After Vaccination.Front Immunol. 2021 Sep 28;12:738123. doi: 10.3389/fimmu.2021.738123. eCollection 2021. Front Immunol. 2021. PMID: 34650561 Free PMC article.
-
Humoral immune responses to infection: common mechanisms and unique strategies to combat pathogen immune evasion tactics.Curr Opin Immunol. 2018 Apr;51:46-54. doi: 10.1016/j.coi.2018.02.001. Epub 2018 Feb 23. Curr Opin Immunol. 2018. PMID: 29477969 Free PMC article. Review.
References
-
- Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18(3):278–285. - PubMed
-
- Gatto D, Brink R. B cell localization: Regulation by EBI2 and its oxysterol ligand. Trends Immunol. 2013;34(7):336–341. - PubMed
-
- MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. - PubMed
-
- Tarlinton D, Good-Jacobson K. Diversity among memory B cells: Origin, consequences, and utility. Science. 2013;341(6151):1205–1211. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

