SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway
- PMID: 25623055
- PMCID: PMC4320842
- DOI: 10.1186/s12943-014-0276-y
SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway
Abstract
Background: Gallbladder cancer (GBC) is a leading cause of cancer-related death worldwide, and its prognosis remains poor, with 5-year survival of approximately 5%. In this study, we analyzed the involvement of a novel proteoglycan, Sparc/osteonectin, cwcv, and kazal-like domains proteoglycan 1 (SPOCK1), in the tumor progression and prognosis of human GBC.
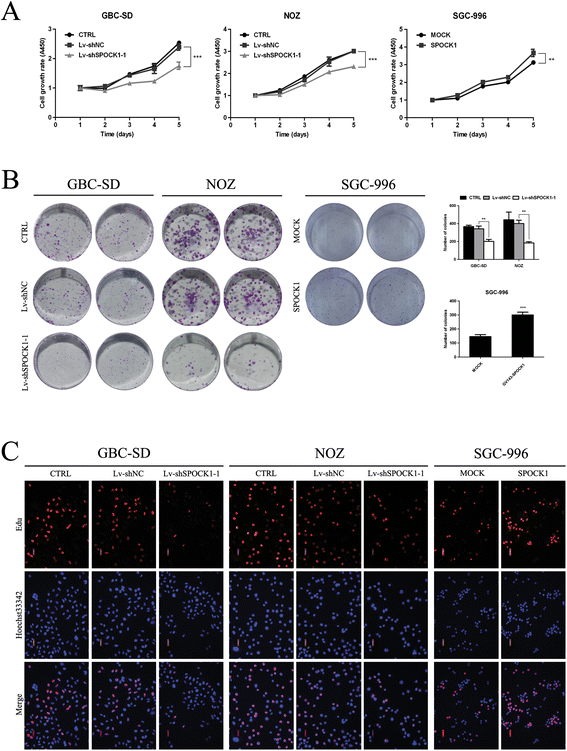
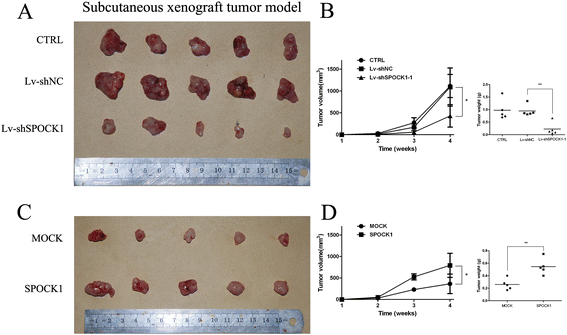
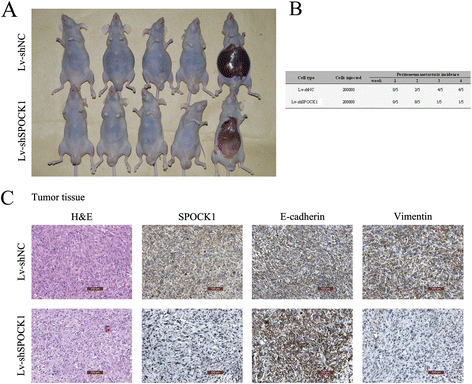
Methods: SPOCK1 expression levels were measured in fresh samples and stored specimens of GBC and adjacent nontumor tissues. The effect of SPOCK1 on cell growth, DNA replication, migration and invasion were explored by Cell Counting Kit-8, colony formation, EdU retention assay, wound healing, and transwell migration assays, flow cytometric analysis, western blotting, and in vivo tumorigenesis and metastasis in nude mice.
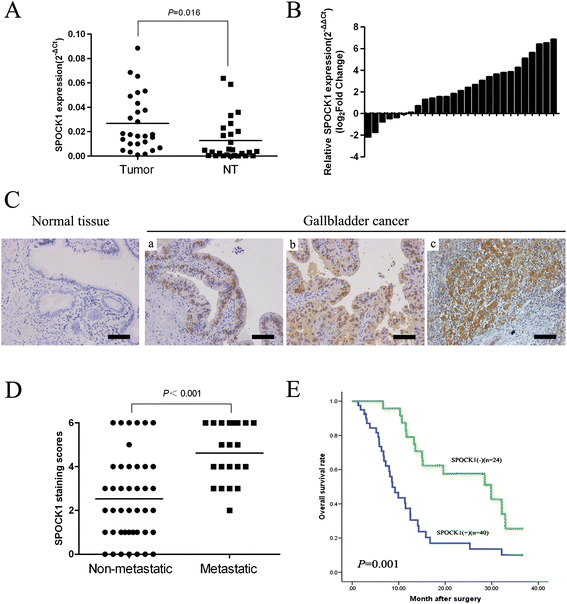
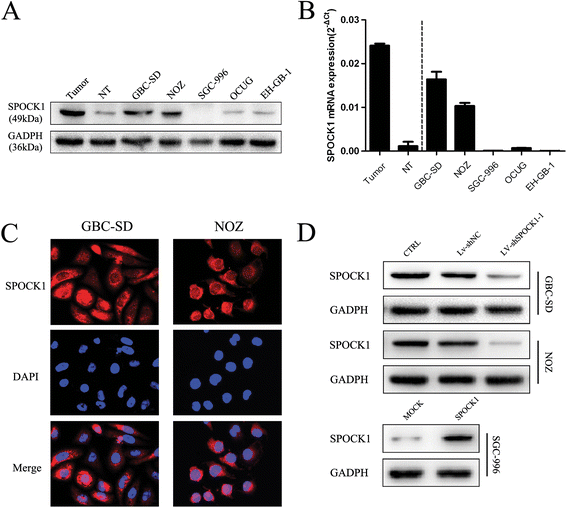
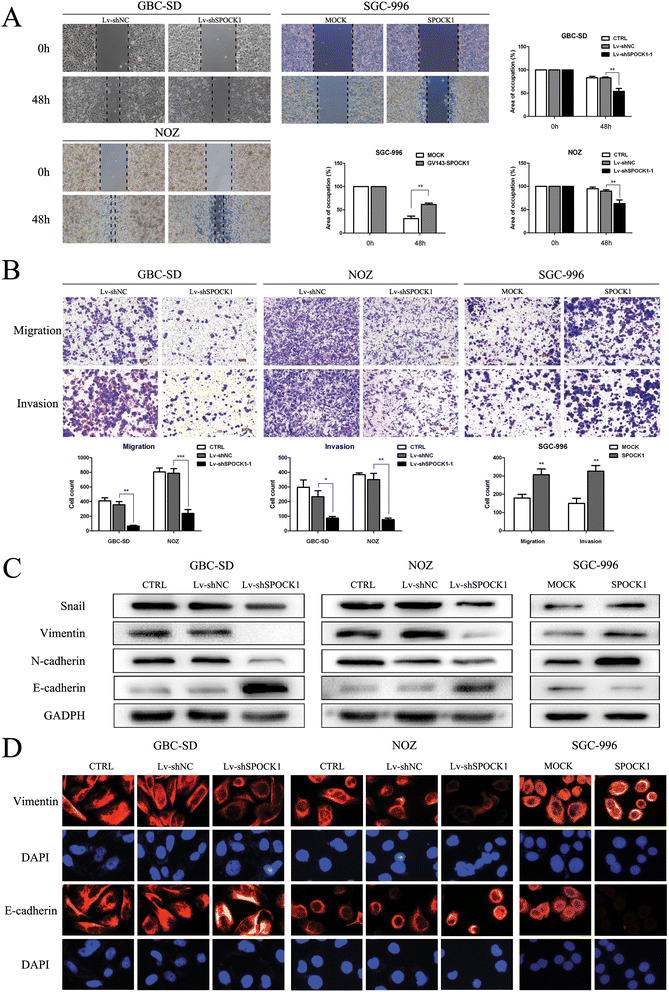
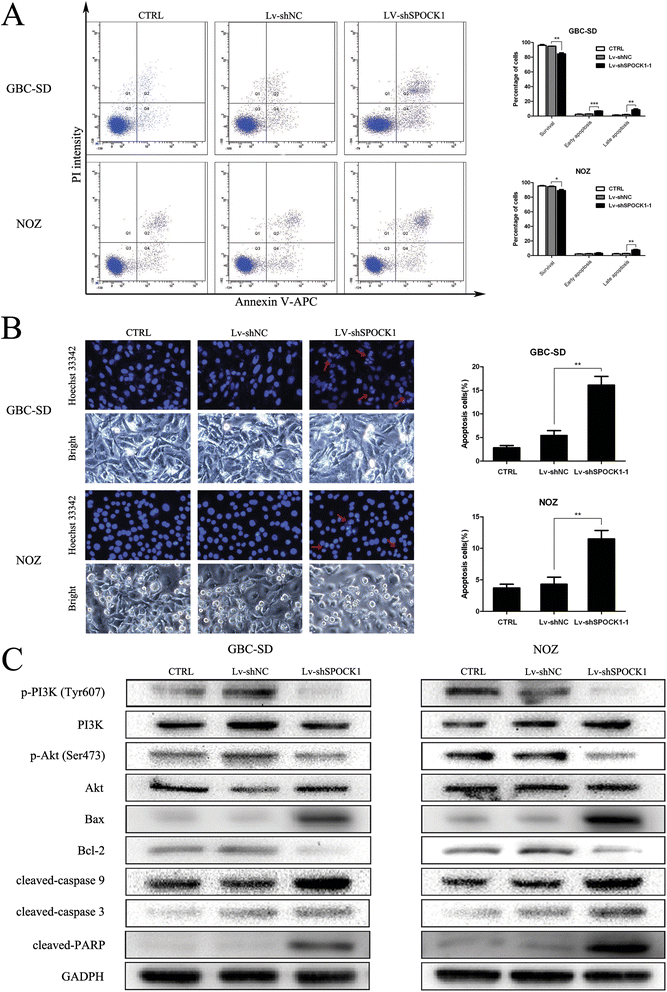
Results: SPOCK1 mRNA and protein levels were increased in human GBC tissues compared with those in nontumor tissues. Immunohistochemical analysis indicated that SPOCK1 levels were increased in tumors that became metastatic, compared with those that did not, which was significantly associated with histological differentiation and patients with shorter overall survival periods. Knockdown of SPOCK1 expression by lentivirus-mediated shRNA transduction resulted in significant inhibition of GBC cell growth, colony formation, DNA replication, and invasion in vitro. The knockdown cells also formed smaller xenografted tumors than control GBC cells in nude mice. Overexpression of SPOCK1 had the opposite effects. In addition, SPOCK1 promoted cancer cell migration and epithelial-mesenchymal transition by regulating the expression of relevant genes. We found that activation of the PI3K/Akt pathway was involved in the oncogenic functions of SPOCK1 in GBC.
Conclusions: SPOCK1 activates PI3K/Akt signaling to block apoptosis and promote proliferation and metastasis by GBC cells in vitro and in vivo. Levels of SPOCK1 increase with the progression of human GBC. SPOCK1 acts as an oncogene and may be a prognostic factor or therapeutic target for patients with GBC.
Figures
Similar articles
-
SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice.Gastroenterology. 2013 Jan;144(1):179-191.e4. doi: 10.1053/j.gastro.2012.09.042. Epub 2012 Sep 25. Gastroenterology. 2013. PMID: 23022495
-
Knockdown of Long Noncoding RNA Urothelial Carcinoma-Associated 1 Represses Gallbladder Cancer Advancement by Regulating SPOCK1 Expression Through Sponging miR-613.Cancer Biother Radiopharm. 2023 Aug;38(6):354-363. doi: 10.1089/cbr.2020.4290. Epub 2020 Oct 22. Cancer Biother Radiopharm. 2023. PMID: 33090888
-
SPOCK1 promotes the proliferation, migration and invasion of glioma cells through PI3K/AKT and Wnt/β-catenin signaling pathways.Oncol Rep. 2016 Jun;35(6):3566-76. doi: 10.3892/or.2016.4757. Epub 2016 Apr 20. Oncol Rep. 2016. PMID: 27108836
-
The emerging role of long non-coding RNA in gallbladder cancer pathogenesis.Biochimie. 2017 Jan;132:152-160. doi: 10.1016/j.biochi.2016.11.007. Epub 2016 Nov 25. Biochimie. 2017. PMID: 27894946 Free PMC article. Review.
-
SPOCK1 Involvement in Epithelial-to-Mesenchymal Transition: A New Target in Cancer Therapy?Cancer Manag Res. 2020 May 18;12:3561-3569. doi: 10.2147/CMAR.S249754. eCollection 2020. Cancer Manag Res. 2020. PMID: 32547193 Free PMC article. Review.
Cited by
-
SPOCK1 promotes tumor growth and metastasis in human prostate cancer.Drug Des Devel Ther. 2016 Jul 18;10:2311-21. doi: 10.2147/DDDT.S91321. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27486308 Free PMC article.
-
miR-101 targeting ZFX suppresses tumor proliferation and metastasis by regulating the MAPK/Erk and Smad pathways in gallbladder carcinoma.Oncotarget. 2016 Apr 19;7(16):22339-54. doi: 10.18632/oncotarget.7970. Oncotarget. 2016. PMID: 26968949 Free PMC article.
-
Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells.Cancer Cell Int. 2017 Jan 5;17:9. doi: 10.1186/s12935-016-0377-3. eCollection 2017. Cancer Cell Int. 2017. PMID: 28070171 Free PMC article.
-
Ring finger protein 125, as a potential highly aggressive and unfavorable prognostic biomarker, promotes the invasion and metastasis of human gallbladder cancers via activating the TGF- β1-SMAD3-ID1 signaling pathway.Oncotarget. 2017 Jul 25;8(30):49897-49914. doi: 10.18632/oncotarget.18180. Oncotarget. 2017. PMID: 28611292 Free PMC article.
-
SPOCK1 Overexpression Confers a Poor Prognosis in Urothelial Carcinoma.J Cancer. 2016 Jan 29;7(4):467-76. doi: 10.7150/jca.13625. eCollection 2016. J Cancer. 2016. PMID: 26918061 Free PMC article.
References
-
- Dong P, Zhang Y, Gu J, Wu W, Li M, Yang J, et al. Wogonin, an active ingredient of Chinese herb medicine Scutellaria baicalensis, inhibits the mobility and invasion of human gallbladder carcinoma GBC-SD cells by inducing the expression of maspin. J Ethnopharmacol. 2011;137:1373–1380. doi: 10.1016/j.jep.2011.08.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

