Chronic filarial infection provides protection against bacterial sepsis by functionally reprogramming macrophages
- PMID: 25611587
- PMCID: PMC4303312
- DOI: 10.1371/journal.ppat.1004616
Chronic filarial infection provides protection against bacterial sepsis by functionally reprogramming macrophages
Abstract
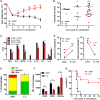
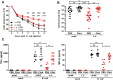
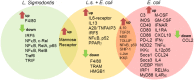
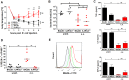
Helminths immunomodulate their hosts and induce a regulatory, anti-inflammatory milieu that prevents allergies and autoimmune diseases. Helminth immunomodulation may benefit sepsis outcome by preventing exacerbated inflammation and severe pathology, but the influence on bacterial clearance remains unclear. To address this, mice were chronically infected with the filarial nematode Litomosoides sigmodontis (L.s.) and the outcome of acute systemic inflammation caused by i.p. Escherichia coli injection was determined. L.s. infection significantly improved E. coli-induced hypothermia, bacterial clearance and sepsis survival and correlated with reduced concentrations of associated pro-inflammatory cytokines/chemokines and a less pronounced pro-inflammatory macrophage gene expression profile. Improved sepsis outcome in L.s.-infected animals was mediated by macrophages, but independent of the alternatively activated macrophage subset. Endosymbiotic Wolbachia bacteria that are present in most human pathogenic filariae, as well as L.s., signal via TLR2 and modulate macrophage function. Here, gene expression profiles of peritoneal macrophages from L.s.-infected mice revealed a downregulation of genes involved in TLR signaling, and pulsing of macrophages in vitro with L.s. extract reduced LPS-triggered activation. Subsequent transfer improved sepsis outcome in naïve mice in a Wolbachia- and TLR2-dependent manner. In vivo, phagocytosis was increased in macrophages from L.s.-infected wild type, but not TLR2-deficient animals. In association, L.s. infection neither improved bacterial clearance in TLR2-deficient animals nor ameliorated E. coli-induced hypothermia and sepsis survival. These results indicate that chronic L.s. infection has a dual beneficial effect on bacterial sepsis, reducing pro-inflammatory immune responses and improving bacterial control. Thus, helminths and their antigens may not only improve the outcome of autoimmune and allergic diseases, but may also present new therapeutic approaches for acute inflammatory diseases that do not impair bacterial control.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Escherichia coli-induced immune paralysis is not exacerbated during chronic filarial infection.Immunology. 2015 May;145(1):150-60. doi: 10.1111/imm.12435. Immunology. 2015. PMID: 25521437 Free PMC article.
-
Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner.J Immunol. 2006 Jul 15;177(2):1240-9. doi: 10.4049/jimmunol.177.2.1240. J Immunol. 2006. PMID: 16818783
-
Patency of Litomosoides sigmodontis infection depends on Toll-like receptor 4 whereas Toll-like receptor 2 signalling influences filarial-specific CD4(+) T-cell responses.Immunology. 2016 Apr;147(4):429-42. doi: 10.1111/imm.12573. Immunology. 2016. PMID: 26714796 Free PMC article.
-
[Bacterial symbionts (Wolbachia) of filarial nematodes: implications for the treatment and pathology of filariasis].Ann Ist Super Sanita. 2001;37(2):265-73. Ann Ist Super Sanita. 2001. PMID: 11758285 Review. Italian.
-
Wolbachia and its implications for the immunopathology of filariasis.Endocr Metab Immune Disord Drug Targets. 2012 Mar;12(1):53-6. doi: 10.2174/187153012799279108. Endocr Metab Immune Disord Drug Targets. 2012. PMID: 22214329 Review.
Cited by
-
Trained immunity in type 2 immune responses.Mucosal Immunol. 2022 Jun;15(6):1158-1169. doi: 10.1038/s41385-022-00557-0. Epub 2022 Sep 5. Mucosal Immunol. 2022. PMID: 36065058 Free PMC article. Review.
-
The immune response of inbred laboratory mice to Litomosoides sigmodontis: A route to discovery in myeloid cell biology.Parasite Immunol. 2020 Jul;42(7):e12708. doi: 10.1111/pim.12708. Epub 2020 Mar 21. Parasite Immunol. 2020. PMID: 32145033 Free PMC article. Review.
-
Recombinant Fasciola hepatica Fatty Acid Binding Protein as a Novel Anti-Inflammatory Biotherapeutic Drug in an Acute Gram-Negative Nonhuman Primate Sepsis Model.Microbiol Spectr. 2021 Dec 22;9(3):e0191021. doi: 10.1128/Spectrum.01910-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937173 Free PMC article.
-
Eosinophils in filarial infections: Inducers of protection or pathology?Front Immunol. 2022 Oct 31;13:983812. doi: 10.3389/fimmu.2022.983812. eCollection 2022. Front Immunol. 2022. PMID: 36389745 Free PMC article. Review.
-
The central adaptor molecule TRIF influences L. sigmodontis worm development.Parasitol Res. 2019 Feb;118(2):539-549. doi: 10.1007/s00436-018-6159-1. Epub 2019 Jan 15. Parasitol Res. 2019. PMID: 30643971
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

