Characterization of a unique cell population marked by transgene expression in the adult cochlea of nestin-CreER(T2)/tdTomato-reporter mice
- PMID: 25611038
- PMCID: PMC4439321
- DOI: 10.1002/cne.23747
Characterization of a unique cell population marked by transgene expression in the adult cochlea of nestin-CreER(T2)/tdTomato-reporter mice
Abstract
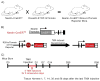
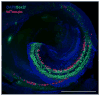
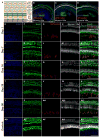
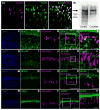
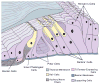
Hair cells in the adult mammalian cochlea cannot spontaneously regenerate after damage, resulting in the permanency of hearing loss. Stem cells have been found to be present in the cochlea of young rodents; however, there has been little evidence for their existence into adulthood. We used nestin-CreER(T2)/tdTomato-reporter mice to trace the lineage of putative nestin-expressing cells and their progeny in the cochleae of adult mice. Nestin, an intermediate filament found in neural progenitor cells during early development and adulthood, is regarded as a multipotent and neural stem cell marker. Other investigators have reported its presence in postnatal and young adult rodents; however, there are discrepancies among these reports. Using lineage tracing, we documented a robust population of tdTomato-expressing cells and evaluated these cells at a series of adult time points. Upon activation of the nestin promoter, tdTomato was observed just below and medial to the inner hair cell layer. All cells colocalized with the stem cell and cochlear-supporting-cell marker Sox2 as well as the supporting cell and Schwann cell marker Sox10; however, they did not colocalize with the Schwann cell marker Krox20, spiral ganglion marker NF200, nor glial fibrillary acidic acid (GFAP)-expressing supporting cell marker. The cellular identity of this unique population of tdTomato-expressing cells in the adult cochlea of nestin-CreER(T2)/tdTomato mice remains unclear; however, these cells may represent a type of supporting cell on the neural aspect of the inner hair cell layer.
Keywords: AB_10013382; AB_10015251; AB_10064079; AB_149792; AB_2195374; AB_2251134; AB_2286684; AB_2314882; AB_306298; AB_396354; inner ear; mouse; regeneration; stem cell; supporting cell.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures

Similar articles
-
Evaluation of Nestin Expression in the Developing and Adult Mouse Inner Ear.Stem Cells Dev. 2016 Oct 1;25(19):1419-32. doi: 10.1089/scd.2016.0176. Epub 2016 Sep 7. Stem Cells Dev. 2016. PMID: 27474107 Free PMC article.
-
Characterizing a novel vGlut3-P2A-iCreER knockin mouse strain in cochlea.Hear Res. 2018 Jul;364:12-24. doi: 10.1016/j.heares.2018.04.006. Epub 2018 Apr 17. Hear Res. 2018. PMID: 29706463
-
Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFAP and nestin promoter-GFP transgenic mice.Gene Expr Patterns. 2011 Jan-Feb;11(1-2):22-32. doi: 10.1016/j.gep.2010.08.008. Epub 2010 Sep 9. Gene Expr Patterns. 2011. PMID: 20817025
-
The potential of nestin-expressing hair follicle stem cells in regenerative medicine.Expert Opin Biol Ther. 2007 Mar;7(3):289-91. doi: 10.1517/14712598.7.3.289. Expert Opin Biol Ther. 2007. PMID: 17309321 Review.
-
The biological strategies for hearing re-establishment based on the stem/progenitor cells.Neurosci Lett. 2019 Oct 15;711:134406. doi: 10.1016/j.neulet.2019.134406. Epub 2019 Aug 1. Neurosci Lett. 2019. PMID: 31377244 Review.
Cited by
-
Persistent myelin abnormalities in a third trimester-equivalent mouse model of fetal alcohol spectrum disorder.Alcohol Clin Exp Res. 2022 Jan;46(1):77-86. doi: 10.1111/acer.14752. Epub 2021 Dec 15. Alcohol Clin Exp Res. 2022. PMID: 34825395 Free PMC article.
-
Ethanol changes Nestin-promoter induced neural stem cells to disturb newborn dendritic spine remodeling in the hippocampus of mice.Neural Regen Res. 2024 Feb;19(2):416-424. doi: 10.4103/1673-5374.379051. Neural Regen Res. 2024. PMID: 37488906 Free PMC article.
-
Endothelial Sphingosine 1‑Phosphate Receptor‑1 Mediates Protection and Recovery from Acute Kidney Injury.J Am Soc Nephrol. 2016 Nov;27(11):3383-3393. doi: 10.1681/ASN.2015080922. Epub 2016 Mar 9. J Am Soc Nephrol. 2016. PMID: 26961351 Free PMC article.
-
Evaluation of Nestin Expression in the Developing and Adult Mouse Inner Ear.Stem Cells Dev. 2016 Oct 1;25(19):1419-32. doi: 10.1089/scd.2016.0176. Epub 2016 Sep 7. Stem Cells Dev. 2016. PMID: 27474107 Free PMC article.
-
Conditional knockout of leptin receptor in neural stem cells leads to obesity in mice and affects neuronal differentiation in the hypothalamus early after birth.Mol Brain. 2020 Aug 3;13(1):109. doi: 10.1186/s13041-020-00647-9. Mol Brain. 2020. PMID: 32746867 Free PMC article.
References
-
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain research bulletin. 2002;57(6):751–758. - PubMed
-
- Breuskin I, Bodson M, Thelen N, Thiry M, Borgs L, Nguyen L, Lefebvre PP, Malgrange B. Sox10 promotes the survival of cochlear progenitors during the establishment of the organ of Corti. Developmental biology. 2009;335(2):327–339. - PubMed
-
- Burns JC, Collado MS, Oliver ER, Corwin JT. Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes. The Journal of comparative neurology. 2013;521(6):1430–1448. - PubMed
-
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of comparative neurology. 2001;435(4):406–417. - PubMed
-
- Carricondo F, Iglesias MC, Rodriguez F, Poch-Broto J, Gil-Loyzaga P. In vitro long-term development of cultured inner ear stem cells of newborn rat. Cell and tissue research. 2010;342(1):13–19. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- P30 HD003352/HD/NICHD NIH HHS/United States
- NIH/NIMH R01MH080434/PHS HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- R01 MH080434/MH/NIMH NIH HHS/United States
- 1 R01 DC013912-01/DC/NIDCD NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- KL2 TR000428/TR/NCATS NIH HHS/United States
- R01 DC013912/DC/NIDCD NIH HHS/United States
- NIH/NIDCD 1 R03 DC012432-01/PHS HHS/United States
- R03 DC012432/DC/NIDCD NIH HHS/United States
- 1UL1RR025011/RR/NCRR NIH HHS/United States
- R01 MH078972/MH/NIMH NIH HHS/United States
- TL1 UL1TR000427/TR/NCATS NIH HHS/United States
- NIH P30 HD003352/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

