Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles
- PMID: 25609950
- PMCID: PMC4294622
- DOI: 10.2147/IJN.S73017
Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles
Abstract
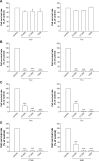
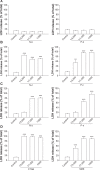
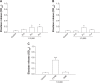
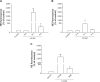
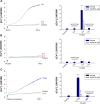
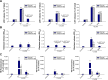
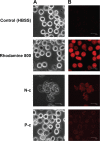
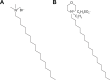
This report compares the effect of lipid and polymeric nanoparticles upon human neutrophils in the presence of cationic surfactants. Nanostructured lipid carriers and poly(lactic-co-glycolic) acid nanoparticles were manufactured as lipid and polymeric systems, respectively. Some cytotoxic and proinflammatory mediators such as lactate dehydrogenase (LDH), elastase, O2(•-), and intracellular Ca(2+) were examined. The nanoparticles showed a size of 170-225 nm. Incorporation of cetyltrimethylammonium bromide or soyaethyl morpholinium ethosulfate, the cationic surfactant, converted zeta potential from a negative to a positive charge. Nanoparticles without cationic surfactants revealed a negligible change on immune and inflammatory responses. Cationic surfactants in both nanoparticulate and free forms induced cell death and the release of mediators. Lipid nanoparticles generally demonstrated a greater response compared to polymeric nanoparticles. The neutrophil morphology observed by electron microscopy confirmed this trend. Cetyltrimethylammonium bromide as the coating material showed more significant activation of neutrophils than soyaethyl morpholinium ethosulfate. Confocal microscope imaging displayed a limited internalization of nanoparticles into neutrophils. It is proposed that cationic nanoparticles interact with the cell membrane, triggering membrane disruption and the following Ca(2+) influx. The elevation of intracellular Ca(2+) induces degranulation and oxidative stress. The consequence of these effects is cytotoxicity and cell death. Caution should be taken when selecting feasible nanoparticulate formulations and cationic additives for consideration of applicability and toxicity.
Keywords: cationic surfactant; inflammation; nanoparticle; neutrophil.
Figures

Similar articles
-
Cationic surfactants in the form of nanoparticles and micelles elicit different human neutrophil responses: a toxicological study.Colloids Surf B Biointerfaces. 2014 Feb 1;114:334-41. doi: 10.1016/j.colsurfb.2013.10.021. Epub 2013 Oct 29. Colloids Surf B Biointerfaces. 2014. PMID: 24246197
-
Cationic liposomes evoke proinflammatory mediator release and neutrophil extracellular traps (NETs) toward human neutrophils.Colloids Surf B Biointerfaces. 2015 Apr 1;128:119-126. doi: 10.1016/j.colsurfb.2015.02.022. Epub 2015 Feb 19. Colloids Surf B Biointerfaces. 2015. PMID: 25731102
-
The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs).Chem Biol Interact. 2015 Jun 25;235:106-14. doi: 10.1016/j.cbi.2015.04.011. Epub 2015 Apr 25. Chem Biol Interact. 2015. PMID: 25920576
-
Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers - a systematic review of in vitro data.Eur J Pharm Biopharm. 2014 May;87(1):1-18. doi: 10.1016/j.ejpb.2014.02.005. Epub 2014 Feb 12. Eur J Pharm Biopharm. 2014. PMID: 24530885 Review.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Cationic Nanomaterials for Autoimmune Diseases Therapy.Front Pharmacol. 2022 Jan 21;12:762362. doi: 10.3389/fphar.2021.762362. eCollection 2021. Front Pharmacol. 2022. PMID: 35126109 Free PMC article. Review.
-
A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris.Molecules. 2022 May 27;27(11):3460. doi: 10.3390/molecules27113460. Molecules. 2022. PMID: 35684396 Free PMC article. Review.
-
Methylation and Esterification of Magnolol for Ameliorating Cutaneous Targeting and Therapeutic Index by Topical Application.Pharm Res. 2016 Sep;33(9):2152-67. doi: 10.1007/s11095-016-1953-x. Epub 2016 May 27. Pharm Res. 2016. PMID: 27233503
-
Polypeptide-Based Systems: From Synthesis to Application in Drug Delivery.Pharmaceutics. 2023 Nov 20;15(11):2641. doi: 10.3390/pharmaceutics15112641. Pharmaceutics. 2023. PMID: 38004619 Free PMC article. Review.
-
Biomaterial-Driven Immunomodulation: Cell Biology-Based Strategies to Mitigate Severe Inflammation and Sepsis.Front Immunol. 2020 Aug 4;11:1726. doi: 10.3389/fimmu.2020.01726. eCollection 2020. Front Immunol. 2020. PMID: 32849612 Free PMC article. Review.
References
-
- Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. - PubMed
-
- Fang CL, Al-Suwayeh SA, Fang JY. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41–55. - PubMed
-
- Tsai MJ, Wu PC, Huang YB, et al. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int J Pharm. 2012;423(2):461–470. - PubMed
-
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

