Acquisition of Relative Interstrand Crosslinker Resistance and PARP Inhibitor Sensitivity in Fanconi Anemia Head and Neck Cancers
- PMID: 25609062
- PMCID: PMC4401632
- DOI: 10.1158/1078-0432.CCR-14-2616
Acquisition of Relative Interstrand Crosslinker Resistance and PARP Inhibitor Sensitivity in Fanconi Anemia Head and Neck Cancers
Abstract
Purpose: Fanconi anemia is an inherited disorder associated with a constitutional defect in the Fanconi anemia DNA repair machinery that is essential for resolution of DNA interstrand crosslinks. Individuals with Fanconi anemia are predisposed to formation of head and neck squamous cell carcinomas (HNSCC) at a young age. Prognosis is poor, partly due to patient intolerance of chemotherapy and radiation requiring dose reduction, which may lead to early recurrence of disease.
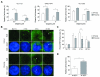
Experimental design: Using HNSCC cell lines derived from the tumors of patients with Fanconi anemia, and murine HNSCC cell lines derived from the tumors of wild-type and Fancc(-/-) mice, we sought to define Fanconi anemia-dependent chemosensitivity and DNA repair characteristics. We utilized DNA repair reporter assays to explore the preference of Fanconi anemia HNSCC cells for non-homologous end joining (NHEJ).
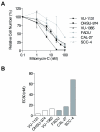
Results: Surprisingly, interstrand crosslinker (ICL) sensitivity was not necessarily Fanconi anemia-dependent in human or murine cell systems. Our results suggest that the increased Ku-dependent NHEJ that is expected in Fanconi anemia cells did not mediate relative ICL resistance. ICL exposure resulted in increased DNA damage sensing and repair by PARP in Fanconi anemia-deficient cells. Moreover, human and murine Fanconi anemia HNSCC cells were sensitive to PARP inhibition, and sensitivity of human cells was attenuated by Fanconi anemia gene complementation.
Conclusions: The observed reliance upon PARP-mediated mechanisms reveals a means by which Fanconi anemia HNSCCs can acquire relative resistance to the ICL-based chemotherapy that is a foundation of HNSCC treatment, as well as a potential target for overcoming chemoresistance in the chemosensitive individual.
©2015 American Association for Cancer Research.
Figures


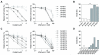
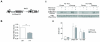
Similar articles
-
EGFR-activating mutations correlate with a Fanconi anemia-like cellular phenotype that includes PARP inhibitor sensitivity.Cancer Res. 2013 Oct 15;73(20):6254-63. doi: 10.1158/0008-5472.CAN-13-0044. Epub 2013 Aug 21. Cancer Res. 2013. PMID: 23966292 Free PMC article.
-
Inhibition of the Nedd8 system sensitizes cells to DNA interstrand cross-linking agents.Mol Cancer Res. 2012 Mar;10(3):369-77. doi: 10.1158/1541-7786.MCR-11-0497. Epub 2012 Jan 4. Mol Cancer Res. 2012. PMID: 22219386 Free PMC article.
-
Fanconi anemia pathway heterogeneity revealed by cisplatin and oxaliplatin treatments.Cancer Lett. 2010 Jun 1;292(1):73-9. doi: 10.1016/j.canlet.2009.11.009. Epub 2010 Jan 19. Cancer Lett. 2010. PMID: 20034732
-
Chromosome Instability in Fanconi Anemia: From Breaks to Phenotypic Consequences.Genes (Basel). 2020 Dec 21;11(12):1528. doi: 10.3390/genes11121528. Genes (Basel). 2020. PMID: 33371494 Free PMC article. Review.
-
Crosstalk between translesion synthesis, Fanconi anemia network, and homologous recombination repair pathways in interstrand DNA crosslink repair and development of chemoresistance.Mutat Res Rev Mutat Res. 2015 Jan-Mar;763:258-66. doi: 10.1016/j.mrrev.2014.11.005. Epub 2014 Nov 20. Mutat Res Rev Mutat Res. 2015. PMID: 25795124 Free PMC article. Review.
Cited by
-
CHK2 activation contributes to the development of oxaliplatin resistance in colorectal cancer.Br J Cancer. 2022 Nov;127(9):1615-1628. doi: 10.1038/s41416-022-01946-9. Epub 2022 Aug 23. Br J Cancer. 2022. PMID: 35999268 Free PMC article.
-
Analysis of PARP inhibitor toxicity by multidimensional fluorescence microscopy reveals mechanisms of sensitivity and resistance.Nat Commun. 2018 Jul 11;9(1):2678. doi: 10.1038/s41467-018-05031-9. Nat Commun. 2018. PMID: 29992957 Free PMC article.
-
Generating New FANCA-Deficient HNSCC Cell Lines by Genomic Editing Recapitulates the Cellular Phenotypes of Fanconi Anemia.Genes (Basel). 2021 Apr 9;12(4):548. doi: 10.3390/genes12040548. Genes (Basel). 2021. PMID: 33918752 Free PMC article.
-
HSF2BP negatively regulates homologous recombination in DNA interstrand crosslink repair.Nucleic Acids Res. 2020 Mar 18;48(5):2442-2456. doi: 10.1093/nar/gkz1219. Nucleic Acids Res. 2020. PMID: 31960047 Free PMC article.
-
Profile of pembrolizumab in the treatment of head and neck squamous cell carcinoma: design development and place in therapy.Drug Des Devel Ther. 2017 Aug 31;11:2537-2549. doi: 10.2147/DDDT.S119537. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28919706 Free PMC article. Review.
References
-
- Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005:2925–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

