SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner
- PMID: 25607651
- PMCID: PMC4614943
- DOI: 10.4161/15384101.2014.980641
SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner
Abstract
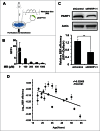
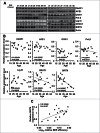
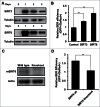
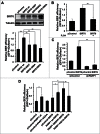
In principle, a decline in base excision repair (BER) efficiency with age should lead to genomic instability and ultimately contribute to the onset of the aging phenotype. Although multiple studies have indicated a negative link between aging and BER, the change of BER efficiency with age in humans has not been systematically analyzed. Here, with foreskin fibroblasts isolated from 19 donors between 20 and 64 y of age, we report a significant decline of BER efficiency with age using a newly developed GFP reactivation assay. We further observed a very strong negative correlation between age and the expression levels of SIRT6, a factor which is known to maintain genomic integrity by improving DNA double strand break (DSB) repair. Our mechanistic study suggests that, similar to the regulatory role that SIRT6 plays in DNA DSB repair, SIRT6 regulates BER in a PARP1-depdendent manner. Moreover, overexpression of SIRT6 rescues the decline of BER in aged fibroblasts. In summary, our results uncovered the regulatory mechanisms of BER by SIRT6, suggesting that SIRT6 reactivation in aging tissues may help delay the process of aging through improving BER.
Keywords: PARP1; SIRT6; SIRTUIN; aging; base excision repair; mono-ADP-ribosylation.
Figures

Similar articles
-
Sirtuin 6 (SIRT6) rescues the decline of homologous recombination repair during replicative senescence.Proc Natl Acad Sci U S A. 2012 Jul 17;109(29):11800-5. doi: 10.1073/pnas.1200583109. Epub 2012 Jul 2. Proc Natl Acad Sci U S A. 2012. PMID: 22753495 Free PMC article.
-
Repairing split ends: SIRT6, mono-ADP ribosylation and DNA repair.Aging (Albany NY). 2011 Sep;3(9):829-35. doi: 10.18632/aging.100389. Aging (Albany NY). 2011. PMID: 21946623 Free PMC article. Review.
-
SIRT6 promotes DNA repair under stress by activating PARP1.Science. 2011 Jun 17;332(6036):1443-6. doi: 10.1126/science.1202723. Science. 2011. PMID: 21680843 Free PMC article.
-
Temporal dynamics of base excision/single-strand break repair protein complex assembly/disassembly are modulated by the PARP/NAD+/SIRT6 axis.Cell Rep. 2021 Nov 2;37(5):109917. doi: 10.1016/j.celrep.2021.109917. Cell Rep. 2021. PMID: 34731617 Free PMC article.
-
NAD+-mediated regulation of mammalian base excision repair.DNA Repair (Amst). 2020 Sep;93:102930. doi: 10.1016/j.dnarep.2020.102930. DNA Repair (Amst). 2020. PMID: 33087267 Free PMC article. Review.
Cited by
-
Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases.Cells. 2018 Dec 12;7(12):268. doi: 10.3390/cells7120268. Cells. 2018. PMID: 30545089 Free PMC article. Review.
-
Sirtuin 6 Overexpression Improves Rotator Cuff Tendon-to-Bone Healing in the Aged.Cells. 2023 Aug 10;12(16):2035. doi: 10.3390/cells12162035. Cells. 2023. PMID: 37626845 Free PMC article.
-
Emerging roles of SIRT6 in human diseases and its modulators.Med Res Rev. 2021 Mar;41(2):1089-1137. doi: 10.1002/med.21753. Epub 2020 Dec 16. Med Res Rev. 2021. PMID: 33325563 Free PMC article. Review.
-
The many faces of SIRT6 in the retina and retinal pigment epithelium.Front Cell Dev Biol. 2023 Nov 1;11:1244765. doi: 10.3389/fcell.2023.1244765. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38016059 Free PMC article. Review.
-
Biological and catalytic functions of sirtuin 6 as targets for small-molecule modulators.J Biol Chem. 2020 Aug 7;295(32):11021-11041. doi: 10.1074/jbc.REV120.011438. Epub 2020 Jun 9. J Biol Chem. 2020. PMID: 32518153 Free PMC article. Review.
References
-
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194-217; PMID:23746838; http://dx.doi.org/10.1016/j.cell.2013.05.039 - DOI - PMC - PubMed
-
- Tigges J, Krutmann J, Fritsche E, Haendeler J, Schaal H, Fischer JW, Kalfalah F, Reinke H, Reifenberger G, Stuhler K, et al. . The hallmarks of fibroblast ageing. Mech Ageing Dev 2014; 138:26-44; PMID:24686308; http://dx.doi.org/10.1016/j.mad.2014.03.004 - DOI - PubMed
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature 1993; 362:709-15; PMID:8469282; http://dx.doi.org/10.1038/362709a0 - DOI - PubMed
-
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009; 30:2-10; PMID:18978338; http://dx.doi.org/10.1093/carcin/bgn250 - DOI - PMC - PubMed
-
- Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Rep 2007; 6:544-59; PMID:17112792; http://dx.doi.org/10.1016/j.dnarep.2006.10.017 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
