Stromal-derived factor-1α/CXCL12-CXCR4 chemotactic pathway promotes perineural invasion in pancreatic cancer
- PMID: 25605248
- PMCID: PMC4467110
- DOI: 10.18632/oncotarget.3069
Stromal-derived factor-1α/CXCL12-CXCR4 chemotactic pathway promotes perineural invasion in pancreatic cancer
Abstract
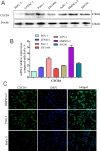
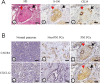
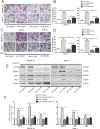
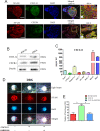
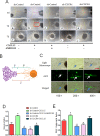
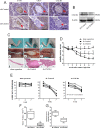
Perineural invasion (PNI) is considered as an alternative route for the metastatic spread of pancreatic cancer cells; however, the molecular changes leading to PNI are still poorly understood. In this study, we show that the CXCL12/CXCR4 axis plays a pivotal role in the neurotropism of pancreatic cancer cells to local peripheral nerves. Immunohistochemical staining results revealed that CXCR4 elevation correlated with PNI in 78 pancreatic cancer samples. Both in vitro and in vivo PNI models were applied to investigate the function of the CXCL12/CXCR4 signaling in PNI progression and pathogenesis. The results showed that the activation of the CXCL12/CXCR4 axis significantly increased pancreatic cancer cells invasion and promoted the outgrowth of the dorsal root ganglia. CXCL12 derived from the peripheral nerves stimulated the invasion and chemotactic migration of CXCR4-positive cancer cells in a paracrine manner, eventually leading to PNI. In vivo analyses revealed that the abrogation of the activated signaling inhibited tumor growth and invasion of the sciatic nerve toward the spinal cord. These data indicate that the CXCL12/CXCR4 axis may be a novel therapeutic target to prevent the perineural dissemination of pancreatic cancer.
Keywords: CXCL12/CXCR4 axis; pancreatic cancer; perineural invasion; tumor microenvironment.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Inflammatory CXCL12-CXCR4/CXCR7 axis mediates G-protein signaling pathway to influence the invasion and migration of nasopharyngeal carcinoma cells.Tumour Biol. 2016 Jun;37(6):8169-79. doi: 10.1007/s13277-015-4686-2. Epub 2015 Dec 29. Tumour Biol. 2016. PMID: 26715277
-
CXCL12-CXCR4 promotes proliferation and invasion of pancreatic cancer cells.Asian Pac J Cancer Prev. 2013;14(9):5403-8. doi: 10.7314/apjcp.2013.14.9.5403. Asian Pac J Cancer Prev. 2013. PMID: 24175834
-
Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer.Clin Cancer Res. 2014 Aug 15;20(16):4326-38. doi: 10.1158/1078-0432.CCR-13-3426. Epub 2014 Jun 19. Clin Cancer Res. 2014. PMID: 24947933
-
Role of CXCL12/CXCR4 signaling axis in pancreatic cancer.Chin Med J (Engl). 2013;126(17):3371-4. Chin Med J (Engl). 2013. PMID: 24033967 Review.
-
The chemokine receptors CXCR4/CXCR7 and their primary heterodimeric ligands CXCL12 and CXCL12/high mobility group box 1 in pancreatic cancer growth and development: finding flow.Pancreas. 2015 May;44(4):528-34. doi: 10.1097/MPA.0000000000000298. Pancreas. 2015. PMID: 25872129 Review.
Cited by
-
Translational pancreatic cancer research: A comparative study on patient-derived xenograft models.World J Gastroenterol. 2018 Feb 21;24(7):794-809. doi: 10.3748/wjg.v24.i7.794. World J Gastroenterol. 2018. PMID: 29467550 Free PMC article.
-
Molecular Imaging with MRI: Potential Application in Pancreatic Cancer.Biomed Res Int. 2015;2015:624074. doi: 10.1155/2015/624074. Epub 2015 Oct 22. Biomed Res Int. 2015. PMID: 26579537 Free PMC article. Review.
-
Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation.Oncotarget. 2017 Jun 28;8(38):63461-63483. doi: 10.18632/oncotarget.18831. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28969005 Free PMC article.
-
Senescent fibroblast-derived Chemerin promotes squamous cell carcinoma migration.Oncotarget. 2016 Dec 13;7(50):83554-83569. doi: 10.18632/oncotarget.13446. Oncotarget. 2016. PMID: 27907906 Free PMC article.
-
Signaling in the microenvironment of pancreatic cancer: Transmitting along the nerve.Pharmacol Ther. 2019 Aug;200:126-134. doi: 10.1016/j.pharmthera.2019.04.010. Epub 2019 Apr 29. Pharmacol Ther. 2019. PMID: 31047906 Free PMC article. Review.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control. 2008;15:308–13. - PubMed
-
- Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77–82. - PubMed
-
- Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

