Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency
- PMID: 25603410
- PMCID: PMC4300181
- DOI: 10.1371/journal.pone.0117098
Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency
Abstract
Objective: Matrix Gla protein (MGP) is reported to inhibit bone morphogenetic protein (BMP) signal transduction. MGP deficiency is associated with medial calcification of the arterial wall, in a process that involves both osteogenic transdifferentiation of vascular smooth muscle cells (VSMCs) and mesenchymal transition of endothelial cells (EndMT). In this study, we investigated the contribution of BMP signal transduction to the medial calcification that develops in MGP-deficient mice.
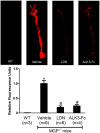
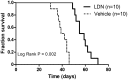
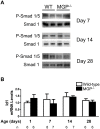
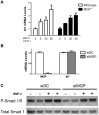
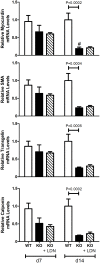
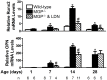
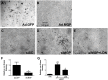
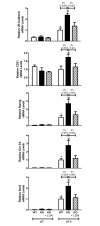
Approach and results: MGP-deficient mice (MGP(-/-)) were treated with one of two BMP signaling inhibitors, LDN-193189 or ALK3-Fc, beginning one day after birth. Aortic calcification was assessed in 28-day-old mice by measuring the uptake of a fluorescent bisphosphonate probe and by staining tissue sections with Alizarin red. Aortic calcification was 80% less in MGP(-/-) mice treated with LDN-193189 or ALK3-Fc compared with vehicle-treated control animals (P<0.001 for both). LDN-193189-treated MGP(-/-) mice survived longer than vehicle-treated MGP(-/-) mice. Levels of phosphorylated Smad1/5 and Id1 mRNA (markers of BMP signaling) did not differ in the aortas from MGP(-/-) and wild-type mice. Markers of EndMT and osteogenesis were increased in MGP(-/-) aortas, an effect that was prevented by LDN-193189. Calcification of isolated VSMCs was also inhibited by LDN-193189.
Conclusions: Inhibition of BMP signaling leads to reduced vascular calcification and improved survival in MGP(-/-) mice. The EndMT and osteogenic transdifferentiation associated with MGP deficiency is dependent upon BMP signaling. These results suggest that BMP signal transduction has critical roles in the development of vascular calcification in MGP-deficient mice.
Conflict of interest statement
Figures


Similar articles
-
Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis.Arterioscler Thromb Vasc Biol. 2012 Mar;32(3):613-22. doi: 10.1161/ATVBAHA.111.242594. Epub 2012 Jan 5. Arterioscler Thromb Vasc Biol. 2012. PMID: 22223731 Free PMC article.
-
Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4.Circ Res. 2008 May 9;102(9):1065-74. doi: 10.1161/CIRCRESAHA.107.166124. Epub 2008 Mar 27. Circ Res. 2008. PMID: 18369157
-
Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells.J Cell Biochem. 2003 Nov 1;90(4):756-65. doi: 10.1002/jcb.10669. J Cell Biochem. 2003. PMID: 14587031
-
Is Matrix Gla Protein Associated with Vascular Calcification? A Systematic Review.Nutrients. 2018 Mar 27;10(4):415. doi: 10.3390/nu10040415. Nutrients. 2018. PMID: 29584693 Free PMC article. Review.
-
Molecular mechanisms mediating vascular calcification: role of matrix Gla protein.Nephrology (Carlton). 2006 Oct;11(5):455-61. doi: 10.1111/j.1440-1797.2006.00660.x. Nephrology (Carlton). 2006. PMID: 17014561 Review.
Cited by
-
Vascular Calcification: New Insights Into BMP Type I Receptor A.Front Pharmacol. 2022 Apr 6;13:887253. doi: 10.3389/fphar.2022.887253. eCollection 2022. Front Pharmacol. 2022. PMID: 35462911 Free PMC article. Review.
-
Matrix Gla Protein Levels Are Associated With Arterial Stiffness and Incident Heart Failure With Preserved Ejection Fraction.Arterioscler Thromb Vasc Biol. 2022 Feb;42(2):e61-e73. doi: 10.1161/ATVBAHA.121.316664. Epub 2021 Nov 23. Arterioscler Thromb Vasc Biol. 2022. PMID: 34809448 Free PMC article.
-
Modulating Cell Fate as a Therapeutic Strategy.Cell Stem Cell. 2018 Sep 6;23(3):329-341. doi: 10.1016/j.stem.2018.05.009. Epub 2018 Jun 14. Cell Stem Cell. 2018. PMID: 29910150 Free PMC article. Review.
-
Strategic Targeting of Multiple BMP Receptors Prevents Trauma-Induced Heterotopic Ossification.Mol Ther. 2017 Aug 2;25(8):1974-1987. doi: 10.1016/j.ymthe.2017.01.008. Epub 2017 Jul 15. Mol Ther. 2017. PMID: 28716575 Free PMC article.
-
Targeting BMP signalling in cardiovascular disease and anaemia.Nat Rev Cardiol. 2016 Feb;13(2):106-20. doi: 10.1038/nrcardio.2015.156. Epub 2015 Oct 13. Nat Rev Cardiol. 2016. PMID: 26461965 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

