Presynaptic inhibition of optogenetically identified VGluT3+ sensory fibres by opioids and baclofen
- PMID: 25599445
- PMCID: PMC4299913
- DOI: 10.1097/01.j.pain.0000460304.63948.40
Presynaptic inhibition of optogenetically identified VGluT3+ sensory fibres by opioids and baclofen
Abstract
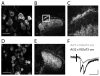
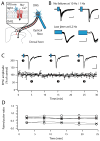
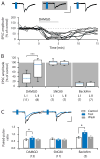
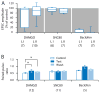
Distinct subsets of sensory nerve fibres are involved in mediating mechanical and thermal pain hypersensitivity. They may also differentially respond to analgesics. Heat-sensitive C-fibres, for example, are thought to respond to μ-opioid receptor (MOR) activation while mechanoreceptive fibres are supposedly sensitive to δ-opioid receptor (DOR) or GABAB receptor (GABABR) activation. The suggested differential distribution of inhibitory neurotransmitter receptors on different subsets of sensory fibres is, however, heavily debated. In this study, we quantitatively compared the degree of presynaptic inhibition exerted by opioids and the GABABR agonist baclofen on (1) vesicular glutamate transporter subtype 3-positive (VGluT3) non-nociceptive primary afferent fibres and (2) putative nociceptive C-fibres. To investigate VGluT3 sensory fibres, we evoked excitatory postsynaptic currents with blue light at the level of the dorsal root ganglion (DRG) in spinal cord slices of mice, expressing channelrhodopsin-2. Putative nociceptive C-fibres were explored in VGluT3-knockout mice through electrical stimulation. The MOR agonist DAMGO strongly inhibited both VGluT3 and VGluT3 C-fibres innervating lamina I neurons but generally had less influence on fibres innervating lamina II neurons. The DOR agonist SNC80 did not have any pronounced effect on synaptic transmission in any fibre type tested. Baclofen, in striking contrast, powerfully inhibited all fibre populations investigated. In summary, we report optogenetic stimulation of DRG neurons in spinal slices as a capable approach for the subtype-selective investigation of primary afferent nerve fibres. Overall, pharmacological accessibility of different subtypes of sensory fibres considerably overlaps, indicating that MOR, DOR, and GABABR expressions are not substantially segregated between heat and mechanosensitive fibres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
GABAB receptors inhibit low-voltage activated and high-voltage activated Ca(2+) channels in sensory neurons via distinct mechanisms.Biochem Biophys Res Commun. 2015 Sep 18;465(2):188-93. doi: 10.1016/j.bbrc.2015.07.137. Epub 2015 Jul 31. Biochem Biophys Res Commun. 2015. PMID: 26239659
-
Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia.Synapse. 2003 Nov;50(2):117-29. doi: 10.1002/syn.10249. Synapse. 2003. PMID: 12923814
-
Low-Threshold Mechanosensitive VGLUT3-Lineage Sensory Neurons Mediate Spinal Inhibition of Itch by Touch.J Neurosci. 2020 Sep 30;40(40):7688-7701. doi: 10.1523/JNEUROSCI.0091-20.2020. Epub 2020 Sep 7. J Neurosci. 2020. PMID: 32895292 Free PMC article.
-
How addictive drugs disrupt presynaptic dopamine neurotransmission.Neuron. 2011 Feb 24;69(4):628-49. doi: 10.1016/j.neuron.2011.02.010. Neuron. 2011. PMID: 21338876 Free PMC article. Review.
-
Optogenetic and Chemogenic Control of Pain Signaling: Molecular Markers.Int J Mol Sci. 2023 Jun 16;24(12):10220. doi: 10.3390/ijms241210220. Int J Mol Sci. 2023. PMID: 37373365 Free PMC article. Review.
Cited by
-
A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour.Int J Mol Sci. 2022 Jan 12;23(2):790. doi: 10.3390/ijms23020790. Int J Mol Sci. 2022. PMID: 35054976 Free PMC article. Review.
-
Optogenetic Activation of Non-Nociceptive Aβ Fibers Induces Neuropathic Pain-Like Sensory and Emotional Behaviors after Nerve Injury in Rats.eNeuro. 2018 Feb 15;5(1):ENEURO.0450-17.2018. doi: 10.1523/ENEURO.0450-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29468190 Free PMC article.
-
Agmatine preferentially antagonizes GluN2B-containing N-methyl-d-aspartate receptors in spinal cord.J Neurophysiol. 2019 Feb 1;121(2):662-671. doi: 10.1152/jn.00172.2018. Epub 2018 Nov 14. J Neurophysiol. 2019. PMID: 30427758 Free PMC article.
-
GABAB receptors-mediated tonic inhibition of glutamate release from Aβ fibers in rat laminae III/IV of the spinal cord dorsal horn.Mol Pain. 2017 Jan-Dec;13:1744806917710041. doi: 10.1177/1744806917710041. Mol Pain. 2017. PMID: 28565998 Free PMC article.
-
Functional remodeling of presynaptic voltage-gated calcium channels in superficial layers of the dorsal horn during neuropathic pain.iScience. 2024 May 14;27(6):109973. doi: 10.1016/j.isci.2024.109973. eCollection 2024 Jun 21. iScience. 2024. PMID: 38827405 Free PMC article.
References
-
- Asrican B, Augustine GJ, Berglund K, Chen S, Chow N, Deisseroth K, Feng G, Gloss B, Hira R, Hoffmann C, Kasai H, Katarya M, Kim J, Kudolo J, Lee LM, Lo SQ, Mancuso J, Matsuzaki M, Nakajima R, Qiu L, Tan G, Tang Y, Ting JT, Tsuda S, Wen L, Zhang X, Zhao S. Next-generation transgenic mice for optogenetic analysis of neural circuits. Front Neural Circuits. 2013;7:160. - PMC - PubMed
-
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. - PubMed
-
- Bao L, Jin S-X, Zhang C, Wang LH, Xu Z-Z, Zhang F-X, Wang L-C, Ning F-S, Cai H-J, Guan J-S, Xiao H-S, Xu Z-Q, He C, Hökfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. - PubMed
-
- Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O’Donnell D, Kieffer BL, Woodbury CJ, Basbaum AI, MacDermott AB, Scherrer G. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 2014;81:1312–1327. - PMC - PubMed
-
- Cahill CM, Holdridge SV, Morinville A. Trafficking of δ-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

