Distinct requirements for HIV-cell fusion and HIV-mediated cell-cell fusion
- PMID: 25589785
- PMCID: PMC4358289
- DOI: 10.1074/jbc.M114.623181
Distinct requirements for HIV-cell fusion and HIV-mediated cell-cell fusion
Abstract
Whether HIV-1 enters cells by fusing with the plasma membrane or with endosomes is a subject of active debate. The ability of HIV-1 to mediate fusion between adjacent cells, a process referred to as "fusion-from-without" (FFWO), shows that this virus can fuse with the plasma membrane. To compare FFWO occurring at the cell surface with HIV-cell fusion through a conventional entry route, we designed an experimental approach that enabled the measurements of both processes in the same sample. The following key differences were observed. First, a very small fraction of viruses fusing with target cells participated in FFWO. Second, whereas HIV-1 fusion with adherent cells was insensitive to actin inhibitors, post-CD4/coreceptor binding steps during FFWO were abrogated. A partial dependence of HIV-cell fusion on actin remodeling was observed in CD4(+) T cells, but this effect appeared to be due to the actin dependence of virus uptake. Third, deletion of the cytoplasmic tail of HIV-1 gp41 dramatically enhanced the ability of the virus to promote FFWO, while having a modest effect on virus-cell fusion. Distinct efficiencies and actin dependences of FFWO versus HIV-cell fusion are consistent with the notion that, except for a minor fraction of particles that mediate fusion between the plasma membranes of adjacent cells, HIV-1 enters through an endocytic pathway. We surmise, however, that cell-cell contacts enabling HIV-1 fusion with the plasma membrane could be favored at the sites of high density of target cells, such as lymph nodes.
Keywords: Cytoskeleton; Endocytosis; Fluorescence; Membrane Fusion; Plasma Membrane.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
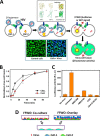
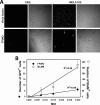
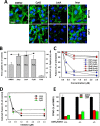
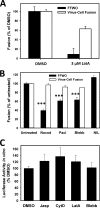
Figures
Similar articles
-
Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion.Retrovirology. 2011 Dec 6;8:99. doi: 10.1186/1742-4690-8-99. Retrovirology. 2011. PMID: 22145853 Free PMC article.
-
HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes.Cell. 2009 May 1;137(3):433-44. doi: 10.1016/j.cell.2009.02.046. Cell. 2009. PMID: 19410541 Free PMC article.
-
Endocytic entry of HIV-1.Curr Biol. 2000 Aug 24;10(16):1005-8. doi: 10.1016/s0960-9822(00)00654-0. Curr Biol. 2000. PMID: 10985390
-
Cell entry of enveloped viruses.Adv Genet. 2011;73:121-83. doi: 10.1016/B978-0-12-380860-8.00004-5. Adv Genet. 2011. PMID: 21310296 Free PMC article. Review.
-
Recent advances in understanding the molecular mechanisms of HIV-1 entry and fusion: revisiting current targets and considering new options for therapeutic intervention.Curr HIV Res. 2004 Jul;2(3):223-34. doi: 10.2174/1570162043351327. Curr HIV Res. 2004. PMID: 15279586 Review.
Cited by
-
Single-cell RNA-seq reveals activation of unique gene groups as a consequence of stem cell-parenchymal cell fusion.Sci Rep. 2016 Mar 21;6:23270. doi: 10.1038/srep23270. Sci Rep. 2016. PMID: 26997336 Free PMC article.
-
A viral fusogen hijacks the actin cytoskeleton to drive cell-cell fusion.Elife. 2020 May 22;9:e51358. doi: 10.7554/eLife.51358. Elife. 2020. PMID: 32441254 Free PMC article.
-
Deletion of ER-retention motif on SARS-CoV-2 spike protein reduces cell hybrid during cell-cell fusion.Cell Biosci. 2021 Jun 23;11(1):114. doi: 10.1186/s13578-021-00626-0. Cell Biosci. 2021. PMID: 34162440 Free PMC article.
-
Insights into the Conformation of the Membrane Proximal Regions Critical to the Trimerization of the HIV-1 gp41 Ectodomain Bound to Dodecyl Phosphocholine Micelles.PLoS One. 2016 Aug 11;11(8):e0160597. doi: 10.1371/journal.pone.0160597. eCollection 2016. PLoS One. 2016. PMID: 27513582 Free PMC article.
-
HIV-2-Infected Macrophages Produce and Accumulate Poorly Infectious Viral Particles.Front Microbiol. 2020 Jul 10;11:1603. doi: 10.3389/fmicb.2020.01603. eCollection 2020. Front Microbiol. 2020. PMID: 32754142 Free PMC article.
References
-
- Gallo S. A., Finnegan C. M., Viard M., Raviv Y., Dimitrov A., Rawat S. S., Puri A., Durell S., Blumenthal R. (2003) The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta 1614, 36–50 - PubMed
-
- Melikyan G. B. (2011) Membrane fusion mediated by human immunodeficiency virus envelope glycoprotein. Curr. Top. Membr. 68, 81–106 - PubMed
-
- Wilen C. B., Tilton J. C., Doms R. W. (2012) Molecular mechanisms of HIV entry. Adv. Exp. Med. Biol. 726, 223–242 - PubMed
-
- Permanyer M., Ballana E., Esté J. A. (2010) Endocytosis of HIV: anything goes. Trends Microbiol. 18, 543–551 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

