Degeneration and regeneration of corneal nerves in response to HSV-1 infection
- PMID: 25587055
- PMCID: PMC4329947
- DOI: 10.1167/iovs.14-15596
Degeneration and regeneration of corneal nerves in response to HSV-1 infection
Abstract
Purpose: Herpes simplex virus type 1 (HSV-1) infection is one cause of neurotrophic keratitis, characterized by decreases in corneal sensation, blink reflex, and tear secretion as consequence of damage to the sensory fibers innervating the cornea. Our aim was to characterize changes in the corneal nerve network and its function in response to HSV-1 infection.
Methods: C57BL/6J mice were infected with HSV-1 or left uninfected. Corneas were harvested at predetermined times post infection (pi) and assessed for β III tubulin, substance P, calcitonin gene-related peptide, and neurofilament H staining by immunohistochemistry (IHC). Corneal sensitivity was evaluated using a Cochet-Bonnet esthesiometer. Expression of genes associated with nerve repair was determined in corneas by real time RT-PCR, Western blotting, and IHC. Semaphorin 7A (SEMA 7A) neutralizing antibody or isotype control was subconjunctivally administered to infected mice.
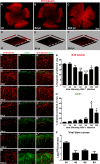
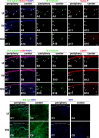
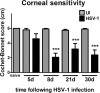
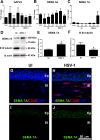
Results: The area of cornea occupied by β III tubulin immunoreactivity and sensitivity significantly decreased by day 8 pi. Modified reinnervation was observed by day 30 pi without recovery of corneal sensation. Sensory fibers were lost by day 8 pi and were still absent or abnormal at day 30 pi. Expression of SEMA 7A increased at day 8 pi, localizing to corneal epithelial cells. Neutralization of SEMA 7A resulted in defective reinnervation and lower corneal sensitivity.
Conclusions: Corneal sensory nerves were lost, consistent with loss of corneal sensation at day 8 pi. At day 30 pi, the cornea reinnervated but without recovering the normal arrangement of its fibers or function. SEMA 7A expression was increased at day 8pi, likely as part of a nerve regeneration mechanism.
Keywords: HSV-1; corneal nerves; neurotrophic keratitis; reinnervation; semaphorin 7A.
Copyright 2015 The Association for Research in Vision and Ophthalmology, Inc.
Figures
Comment in
-
Semaphorin 7a in Herpetic Neurotrophic Keratitis.Invest Ophthalmol Vis Sci. 2015 Feb;56(2):1108. doi: 10.1167/iovs.15-16473. Invest Ophthalmol Vis Sci. 2015. PMID: 26241179 No abstract available.
Similar articles
-
Semaphorin 7a in Herpetic Neurotrophic Keratitis.Invest Ophthalmol Vis Sci. 2015 Feb;56(2):1108. doi: 10.1167/iovs.15-16473. Invest Ophthalmol Vis Sci. 2015. PMID: 26241179 No abstract available.
-
A Central Role for Sympathetic Nerves in Herpes Stromal Keratitis in Mice.Invest Ophthalmol Vis Sci. 2016 Apr;57(4):1749-56. doi: 10.1167/iovs.16-19183. Invest Ophthalmol Vis Sci. 2016. PMID: 27070108 Free PMC article.
-
IL-6 Contributes to Corneal Nerve Degeneration after Herpes Simplex Virus Type I Infection.Am J Pathol. 2016 Oct;186(10):2665-78. doi: 10.1016/j.ajpath.2016.06.007. Epub 2016 Aug 3. Am J Pathol. 2016. PMID: 27497323 Free PMC article.
-
Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation.Ocul Surf. 2019 Jan;17(1):40-49. doi: 10.1016/j.jtos.2018.10.002. Epub 2018 Oct 11. Ocul Surf. 2019. PMID: 30317007 Free PMC article. Review.
-
[Battle with herpes for 37 years].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
Cited by
-
Distinguishing Features of High- and Low-Dose Vaccine against Ocular HSV-1 Infection Correlates with Recognition of Specific HSV-1-Encoded Proteins.Immunohorizons. 2020 Oct 9;4(10):608-626. doi: 10.4049/immunohorizons.2000060. Immunohorizons. 2020. PMID: 33037098 Free PMC article.
-
Antibiotic-Induced Dysbiosis of Gut Microbiota Impairs Corneal Nerve Regeneration by Affecting CCR2-Negative Macrophage Distribution.Am J Pathol. 2018 Dec;188(12):2786-2799. doi: 10.1016/j.ajpath.2018.08.009. Am J Pathol. 2018. PMID: 30470496 Free PMC article.
-
Immunity and pain in the eye: focus on the ocular surface.Clin Exp Immunol. 2022 Apr 4;207(2):149-163. doi: 10.1093/cei/uxab032. Clin Exp Immunol. 2022. PMID: 35020868 Free PMC article. Review.
-
A Highly Efficacious Herpes Simplex Virus 1 Vaccine Blocks Viral Pathogenesis and Prevents Corneal Immunopathology via Humoral Immunity.J Virol. 2016 May 12;90(11):5514-5529. doi: 10.1128/JVI.00517-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27030264 Free PMC article.
-
TRPV1+ sensory nerves modulate corneal inflammation after epithelial abrasion via RAMP1 and SSTR5 signaling.Mucosal Immunol. 2022 May;15(5):867-881. doi: 10.1038/s41385-022-00533-8. Epub 2022 Jun 9. Mucosal Immunol. 2022. PMID: 35680973
References
-
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003; 76: 521–542. - PubMed
-
- Radner W, Mallinger R. Interlacing of collagen lamellae in the midstroma of the human cornea. Cornea. 2002; 21: 598–601. - PubMed
-
- Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980; 69: 196–201. - PubMed
-
- Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010; 90: 478–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

