Tumor promoter-induced cellular senescence: cell cycle arrest followed by geroconversion
- PMID: 25587030
- PMCID: PMC4350340
- DOI: 10.18632/oncotarget.3011
Tumor promoter-induced cellular senescence: cell cycle arrest followed by geroconversion
Abstract
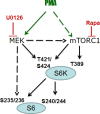
Phorbol ester (PMA or TPA), a tumor promoter, can cause either proliferation or cell cycle arrest, depending on cellular context. For example, in SKBr3 breast cancer cells, PMA hyper-activates the MEK/MAPK pathway, thus inducing p21 and cell cycle arrest. Here we showed that PMA-induced arrest was followed by conversion to cellular senescence (geroconversion). Geroconversion was associated with active mTOR and S6 kinase (S6K). Rapamycin suppressed geroconversion, maintaining quiescence instead. In this model, PMA induced arrest (step one of a senescence program), whereas constitutively active mTOR drove geroconversion (step two). Without affecting Akt phosphorylation, PMA increased phosphorylation of S6K (T389) and S6 (S240/244), and that was completely prevented by rapamycin. Yet, T421/S424 and S235/236 (p-S6K and p-S6, respectively) phosphorylation became rapamycin-insensitive in the presence of PMA. Either MEK or mTOR was sufficient to phosphorylate these PMA-induced rapamycin-resistant sites because co-treatment with U0126 and rapamycin was required to abrogate them. We next tested whether activation of rapamycin-insensitive pathways would shift quiescence towards senescence. In HT-p21 cells, cell cycle arrest was caused by IPTG-inducible p21 and was spontaneously followed by mTOR-dependent geroconversion. Rapamycin suppressed geroconversion, whereas PMA partially counteracted the effect of rapamycin, revealing the involvement of rapamycin-insensitive gerogenic pathways. In normal RPE cells arrested by serum withdrawal, the mTOR/pS6 pathway was inhibited and cells remained quiescent. PMA transiently activated mTOR, enabling partial geroconversion. We conclude that PMA can initiate a senescent program by either inducing arrest or fostering geroconversion or both. Rapamycin can decrease gero-conversion by PMA, without preventing PMA-induced arrest. The tumor promoter PMA is a gero-promoter, which may be useful to study aging in mammals.
Conflict of interest statement
No conflict to declare.
Figures






Similar articles
-
Rapamycin, proliferation and geroconversion to senescence.Cell Cycle. 2018;17(24):2655-2665. doi: 10.1080/15384101.2018.1554781. Epub 2018 Dec 12. Cell Cycle. 2018. PMID: 30541374 Free PMC article.
-
Dual mTORC1/C2 inhibitors suppress cellular geroconversion (a senescence program).Oncotarget. 2015 Sep 15;6(27):23238-48. doi: 10.18632/oncotarget.4836. Oncotarget. 2015. PMID: 26177051 Free PMC article.
-
Hypoxia suppresses conversion from proliferative arrest to cellular senescence.Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13314-8. doi: 10.1073/pnas.1205690109. Epub 2012 Jul 30. Proc Natl Acad Sci U S A. 2012. PMID: 22847439 Free PMC article.
-
Geroconversion: irreversible step to cellular senescence.Cell Cycle. 2014;13(23):3628-35. doi: 10.4161/15384101.2014.985507. Cell Cycle. 2014. PMID: 25483060 Free PMC article. Review.
-
Cell senescence, rapamycin and hyperfunction theory of aging.Cell Cycle. 2022 Jul;21(14):1456-1467. doi: 10.1080/15384101.2022.2054636. Epub 2022 Mar 31. Cell Cycle. 2022. PMID: 35358003 Free PMC article. Review.
Cited by
-
Cellular senescence: when growth stimulation meets cell cycle arrest.Aging (Albany NY). 2023 Feb 19;15(4):905-913. doi: 10.18632/aging.204543. Epub 2023 Feb 19. Aging (Albany NY). 2023. PMID: 36805938 Free PMC article.
-
Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes.Oncotarget. 2016 May 10;7(19):26966-78. doi: 10.18632/oncotarget.9034. Oncotarget. 2016. PMID: 27127181 Free PMC article.
-
Apoptotic effects of high-dose rapamycin occur in S-phase of the cell cycle.Cell Cycle. 2015;14(14):2285-92. doi: 10.1080/15384101.2015.1046653. Epub 2015 May 6. Cell Cycle. 2015. PMID: 25945415 Free PMC article.
-
Early Changes in [18F]FDG Uptake as a Readout for PI3K/Akt/mTOR Targeted Drugs in HER-2-Positive Cancer Xenografts.Mol Imaging. 2021 May 25;2021:5594514. doi: 10.1155/2021/5594514. eCollection 2021. Mol Imaging. 2021. PMID: 34113218 Free PMC article.
-
Delineation of proteome changes driven by cell size and growth rate.Front Cell Dev Biol. 2022 Sep 5;10:980721. doi: 10.3389/fcell.2022.980721. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36133920 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

