Intermediate-conductance calcium-activated potassium channel KCa3.1 and chloride channel modulate chemokine ligand (CCL19/CCL21)-induced migration of dendritic cells
- PMID: 25583444
- PMCID: PMC4458411
- DOI: 10.1016/j.trsl.2014.11.010
Intermediate-conductance calcium-activated potassium channel KCa3.1 and chloride channel modulate chemokine ligand (CCL19/CCL21)-induced migration of dendritic cells
Abstract
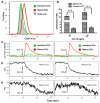
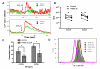
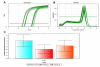
The role of ion channels is largely unknown in chemokine-induced migration in nonexcitable cells such as dendritic cells (DCs). Here, we examined the role of intermediate-conductance calcium-activated potassium channel (KCa3.1) and chloride channel (CLC3) in lymphatic chemokine-induced migration of DCs. The amplitude and kinetics of chemokine ligand (CCL19/CCL21)-induced Ca(2+) influx were associated with chemokine receptor 7 expression levels, extracellular-free Ca(2+) and Cl(-), and independent of extracellular K(+). Chemokines (CCL19 and CCL21) and KCa3.1 activator (1-ethyl-1,3-dihydro-2H-benzimidazol-2-one) induced plasma membrane hyperpolarization and K(+) efflux, which was blocked by 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole, suggesting that KCa3.1 carried larger conductance than the inward calcium release-activated calcium channel. Blockade of KCa3.1, low Cl(-) in the medium, and low dose of 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS) impaired CCL19/CCL21-induced Ca(2+) influx, cell volume change, and DC migration. High doses of DIDS completely blocked DC migration possibly by significantly disrupting mitochondrial membrane potential. In conclusion, KCa3.1 and CLC3 are critical in human DC migration by synergistically regulating membrane potential, chemokine-induced Ca(2+) influx, and cell volume.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Bharadwaj AS, Agrawal DK. Transcription factors in the control of dendritic cell life cycle. Immunologic research. 2007;37:79–96. - PubMed
-
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nature immunology. 2011;12:879–87. - PubMed
-
- Ricart BG, John B, Lee D, Hunter CA, Hammer DA. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. Journal of immunology. 2011;186:53–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

