Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras
- PMID: 25575514
- PMCID: PMC4346775
- DOI: 10.1152/ajplung.00227.2014
Airway responsiveness in CD38-deficient mice in allergic airway disease: studies with bone marrow chimeras
Abstract
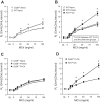
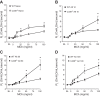
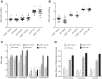
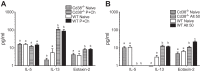
CD38 is a cell-surface protein involved in calcium signaling and contractility of airway smooth muscle. It has a role in normal airway responsiveness and in airway hyperresponsiveness (AHR) developed following airway exposure to IL-13 and TNF-α but appears not to be critical to airway inflammation in response to the cytokines. CD38 is also involved in T cell-mediated immune response to protein antigens. In this study, we assessed the contribution of CD38 to AHR and inflammation to two distinct allergens, ovalbumin and the epidemiologically relevant environmental fungus Alternaria. We also generated bone marrow chimeras to assess whether Cd38(+/+) inflammatory cells would restore AHR in the CD38-deficient (Cd38(-/-)) hosts following ovalbumin challenge. Results show that wild-type (WT) mice develop greater AHR to inhaled methacholine than Cd38(-/-) mice following challenge with either allergen, with comparable airway inflammation. Reciprocal bone marrow transfers did not change the native airway phenotypic differences between WT and Cd38(-/-) mice, indicating that the lower airway reactivity of Cd38(-/-) mice stems from Cd38(-/-) lung parenchymal cells. Following bone marrow transfer from either source and ovalbumin challenge, the phenotype of Cd38(-/-) hosts was partially reversed, whereas the airway phenotype of the WT hosts was preserved. Airway inflammation was similar in Cd38(-/-) and WT chimeras. These results indicate that loss of CD38 on hematopoietic cells is not sufficient to prevent AHR and that the magnitude of airway inflammation is not the predominant underlying determinant of AHR in mice.
Keywords: Alternaria; CD38; allergic airway disease; bone marrow chimeras.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Role of CD38 in TNF-alpha-induced airway hyperresponsiveness.Am J Physiol Lung Cell Mol Physiol. 2008 Feb;294(2):L290-9. doi: 10.1152/ajplung.00367.2007. Epub 2007 Nov 30. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18055841
-
CD38 plays a dual role in allergen-induced airway hyperresponsiveness.Am J Respir Cell Mol Biol. 2009 Apr;40(4):433-42. doi: 10.1165/rcmb.2007-0392OC. Epub 2008 Oct 17. Am J Respir Cell Mol Biol. 2009. PMID: 18931329 Free PMC article.
-
CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge.Am J Physiol Lung Cell Mol Physiol. 2006 Dec;291(6):L1286-93. doi: 10.1152/ajplung.00187.2006. Epub 2006 Aug 4. Am J Physiol Lung Cell Mol Physiol. 2006. PMID: 16891391
-
CD38 and airway hyper-responsiveness: studies on human airway smooth muscle cells and mouse models.Can J Physiol Pharmacol. 2015 Feb;93(2):145-53. doi: 10.1139/cjpp-2014-0410. Epub 2014 Dec 9. Can J Physiol Pharmacol. 2015. PMID: 25594684 Free PMC article. Review.
-
CD38 in the pathogenesis of allergic airway disease: Potential therapeutic targets.Pharmacol Ther. 2017 Apr;172:116-126. doi: 10.1016/j.pharmthera.2016.12.002. Epub 2016 Dec 7. Pharmacol Ther. 2017. PMID: 27939939 Free PMC article. Review.
Cited by
-
CD31+, CD38+, CD44+, and CD103+ lymphocytes in peripheral blood, bronchoalveolar lavage fluid and lung biopsy tissue in sarcoid patients and controls.J Thorac Dis. 2021 Apr;13(4):2300-2318. doi: 10.21037/jtd-20-2396. J Thorac Dis. 2021. PMID: 34012580 Free PMC article.
-
Edaravone attenuates experimental asthma in mice through induction of HO-1 and the Keap1/Nrf2 pathway.Exp Ther Med. 2020 Feb;19(2):1407-1416. doi: 10.3892/etm.2019.8351. Epub 2019 Dec 19. Exp Ther Med. 2020. PMID: 32010316 Free PMC article.
-
Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma.Int J Mol Sci. 2023 Mar 28;24(7):6347. doi: 10.3390/ijms24076347. Int J Mol Sci. 2023. PMID: 37047327 Free PMC article. Review.
-
The Good, the Bad and the Unknown of CD38 in the Metabolic Microenvironment and Immune Cell Functionality of Solid Tumors.Cells. 2019 Dec 24;9(1):52. doi: 10.3390/cells9010052. Cells. 2019. PMID: 31878283 Free PMC article. Review.
-
CD38 plays an age-related role in cholinergic deregulation of airway smooth muscle contractility.J Allergy Clin Immunol. 2022 May;149(5):1643-1654.e8. doi: 10.1016/j.jaci.2021.10.033. Epub 2021 Nov 18. J Allergy Clin Immunol. 2022. PMID: 34800431 Free PMC article.
References
-
- Ammit AJ, Hoffman RK, Amrani Y, Lazaar AL, Hay DW, Torphy TJ, Penn RB, Panettieri RA Jr. Tumor necrosis factor-alpha-induced secretion of RANTES and interleukin-6 from human airway smooth-muscle cells. Modulation by cyclic adenosine monophosphate. Am J Respir Cell Mol Biol 23: 794–802, 2000. - PubMed
-
- Berkman N, Krishnan VL, Gilbey T, Newton R, O'Connor B, Barnes PJ, Chung KF. Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am J Respir Crit Care Med 154: 1804–1811, 1996. - PubMed
-
- Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 171: 1103–1108, 2005. - PubMed
-
- Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol 113: 227–234, 2004. - PubMed
-
- Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, Murray R, Schuber F, Howard MC. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 92: 1324–1333, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

