Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling
- PMID: 25574839
- PMCID: PMC4319420
- DOI: 10.1172/JCI77186
Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling
Abstract
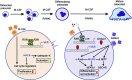
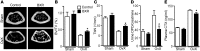
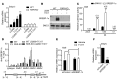
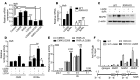
Osteoclasts are bone-resorbing cells that are important for maintenance of bone remodeling and mineral homeostasis. Regulation of osteoclast differentiation and activity is important for the pathogenesis and treatment of diseases associated with bone loss. Here, we demonstrate that retinoid X receptors (RXRs) are key elements of the transcriptional program of differentiating osteoclasts. Loss of RXR function in hematopoietic cells resulted in formation of giant, nonresorbing osteoclasts and increased bone mass in male mice and protected female mice from bone loss following ovariectomy, which induces osteoporosis in WT females. The increase in bone mass associated with RXR deficiency was due to lack of expression of the RXR-dependent transcription factor v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B (MAFB) in osteoclast progenitors. Evaluation of osteoclast progenitor cells revealed that RXR homodimers directly target and bind to the Mafb promoter, and this interaction is required for proper osteoclast proliferation, differentiation, and activity. Pharmacological activation of RXRs inhibited osteoclast differentiation due to the formation of RXR/liver X receptor (LXR) heterodimers, which induced expression of sterol regulatory element binding protein-1c (SREBP-1c), resulting in indirect MAFB upregulation. Our study reveals that RXR signaling mediates bone homeostasis and suggests that RXRs have potential as targets for the treatment of bone pathologies such as osteoporosis.
Figures


Similar articles
-
The multi-faceted role of retinoid X receptor in bone remodeling.Cell Mol Life Sci. 2017 Jun;74(12):2135-2149. doi: 10.1007/s00018-017-2458-4. Epub 2017 Jan 19. Cell Mol Life Sci. 2017. PMID: 28105491 Free PMC article. Review.
-
Structure basis of bigelovin as a selective RXR agonist with a distinct binding mode.J Mol Biol. 2011 Mar 18;407(1):13-20. doi: 10.1016/j.jmb.2011.01.032. Epub 2011 Jan 22. J Mol Biol. 2011. PMID: 21262235
-
Tributyltin induces distinct effects on cortical and trabecular bone in female C57Bl/6J mice.J Cell Physiol. 2018 Sep;233(9):7007-7021. doi: 10.1002/jcp.26495. Epub 2018 Mar 25. J Cell Physiol. 2018. PMID: 29380368 Free PMC article.
-
Molecular determinants of the interactions between SRC-1 and LXR/RXR heterodimers.FEBS Lett. 2010 Sep 24;584(18):3862-6. doi: 10.1016/j.febslet.2010.07.056. Epub 2010 Aug 1. FEBS Lett. 2010. PMID: 20682316
-
Modulation of RXR function through ligand design.Biochim Biophys Acta. 2012 Jan;1821(1):57-69. doi: 10.1016/j.bbalip.2011.04.003. Epub 2011 Apr 16. Biochim Biophys Acta. 2012. PMID: 21515403 Review.
Cited by
-
Bhlhe40 and Bhlhe41 transcription factors regulate alveolar macrophage self-renewal and identity.EMBO J. 2019 Oct 1;38(19):e101233. doi: 10.15252/embj.2018101233. Epub 2019 Aug 15. EMBO J. 2019. PMID: 31414712 Free PMC article.
-
Role of MafB in macrophages.Exp Anim. 2020 Jan 29;69(1):1-10. doi: 10.1538/expanim.19-0076. Epub 2019 Oct 1. Exp Anim. 2020. PMID: 31582643 Free PMC article. Review.
-
Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms.Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. Epub 2015 May 18. Mediators Inflamm. 2015. PMID: 26089604 Free PMC article. Review.
-
Exposure to the RXR Agonist SR11237 in Early Life Causes Disturbed Skeletal Morphogenesis in a Rat Model.Int J Mol Sci. 2019 Oct 20;20(20):5198. doi: 10.3390/ijms20205198. Int J Mol Sci. 2019. PMID: 31635173 Free PMC article.
-
Understanding the Biology of Self-Renewing Macrophages.Cells. 2018 Aug 9;7(8):103. doi: 10.3390/cells7080103. Cells. 2018. PMID: 30096862 Free PMC article. Review.
References
-
- Vanderschueren D, Boonen S, Bouillon R. Physiology of calcium homeostasis and bone remodeling. In: Geusens P, Sambrook P, Lindsay R, eds. Osteoporosis in Clinical Practice: A Practical Guide for Diagnosis and Treatment. 2nd ed. London, United Kingdom: Springer London; 2004:9–14.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

