Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib
- PMID: 25573991
- PMCID: PMC4467871
- DOI: 10.1182/blood-2014-09-603670
Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib
Abstract
Ibrutinib is a Bruton tyrosine kinase inhibitor approved for the treatment of patients with relapsed refractory chronic lymphocytic leukemia (RR-CLL). We describe the characteristics, causes of discontinuation, and outcomes in patients who discontinued treatment with ibrutinib. One hundred twenty-seven patients were enrolled in various clinical trials of ibrutinib, with or without rituximab, at our center. Thirty-three (26%) patients have discontinued ibrutinib to date. The majority of those patients had high-risk features: 94% with unmutated immunoglobulin heavy chain variable gene rearrangement, 58% with del(17p) by fluorescence in situ hybridization, and 54% with a complex karyotype. Causes of discontinuation were disease transformation (7), progressive CLL (7), stem cell transplantation (3), adverse events (11), serious adverse events/deaths (3), and miscellaneous reasons (2). Twenty five patients (76%) died after discontinuing ibrutinib; the median overall survival was 3.1 months after discontinuation. Most patients with RR-CLL who discontinued ibrutinib early were difficult to treat and had poor outcomes.
© 2015 by The American Society of Hematology.
Figures
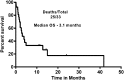
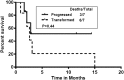
Comment in
-
Life after ibrutinib? A new unmet need in CLL.Blood. 2015 Mar 26;125(13):2013-4. doi: 10.1182/blood-2015-02-622472. Blood. 2015. PMID: 25814485
Similar articles
-
Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia.JAMA Oncol. 2015 Apr;1(1):80-7. doi: 10.1001/jamaoncol.2014.218. JAMA Oncol. 2015. PMID: 26182309 Free PMC article.
-
Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib.Cancer. 2017 Jun 15;123(12):2268-2273. doi: 10.1002/cncr.30596. Epub 2017 Feb 7. Cancer. 2017. PMID: 28171709 Free PMC article.
-
Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group.Haematologica. 2016 Dec;101(12):1573-1580. doi: 10.3324/haematol.2016.144576. Epub 2016 May 19. Haematologica. 2016. PMID: 27198718 Free PMC article.
-
Ibrutinib for treatment of chronic lymphocytic leukemia.Am J Health Syst Pharm. 2016 Mar 15;73(6):367-75. doi: 10.2146/ajhp140760. Am J Health Syst Pharm. 2016. PMID: 26953281 Review.
-
Ibrutinib: a review of its use in patients with mantle cell lymphoma or chronic lymphocytic leukaemia.Drugs. 2015 May;75(7):769-76. doi: 10.1007/s40265-015-0380-3. Drugs. 2015. PMID: 25802231 Review.
Cited by
-
Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects.Front Cell Dev Biol. 2021 Mar 11;9:630942. doi: 10.3389/fcell.2021.630942. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777941 Free PMC article. Review.
-
Long-term Follow-up of Treatment with Ibrutinib and Rituximab in Patients with High-Risk Chronic Lymphocytic Leukemia.Clin Cancer Res. 2017 May 1;23(9):2154-2158. doi: 10.1158/1078-0432.CCR-16-1948. Epub 2016 Oct 19. Clin Cancer Res. 2017. PMID: 27797975 Free PMC article. Clinical Trial.
-
B-cell-specific IRF4 deletion accelerates chronic lymphocytic leukemia development by enhanced tumor immune evasion.Blood. 2019 Nov 14;134(20):1717-1729. doi: 10.1182/blood.2019000973. Blood. 2019. PMID: 31537531 Free PMC article.
-
Clonal dynamics in chronic lymphocytic leukemia.Blood Adv. 2019 Nov 26;3(22):3759-3769. doi: 10.1182/bloodadvances.2019000367. Blood Adv. 2019. PMID: 31770443 Free PMC article.
-
Outcomes with ibrutinib by line of therapy and post-ibrutinib discontinuation in patients with chronic lymphocytic leukemia: Phase 3 analysis.Am J Hematol. 2019 May;94(5):554-562. doi: 10.1002/ajh.25436. Epub 2019 Mar 8. Am J Hematol. 2019. PMID: 30767298 Free PMC article. Clinical Trial.
References
-
- Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14(4):219–232. - PubMed
-
- O'Brien S, Furman RR, Fowler N, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) Monotherapy Demonstrates Long-Term Safety and Durability Of Response In Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) Patients In An Open-Label Extension Study. Blood. 2013;122(21):4163. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

