Controlling ionotropic and metabotropic glutamate receptors with light: principles and potential
- PMID: 25573450
- PMCID: PMC4318769
- DOI: 10.1016/j.coph.2014.12.008
Controlling ionotropic and metabotropic glutamate receptors with light: principles and potential
Abstract
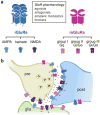
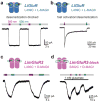
Light offers unique advantages for studying and manipulating biomolecules and the cellular processes that they control. Optical control of ionotropic and metabotropic glutamate receptors has garnered significant interest, since these receptors are central to signaling at neuronal synapses and only optical approaches provide the spatial and temporal resolution required to directly probe receptor function in cells and tissue. Following the classical method of glutamate photo-uncaging, recently developed methods have added other forms of remote control, including those with high molecular specificity and genetic targeting. These tools open the door to the direct optical control of synaptic transmission and plasticity, as well as the probing of native receptor function in intact neural circuits.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


Similar articles
-
Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert.Neuron. 2018 Jun 27;98(6):1080-1098. doi: 10.1016/j.neuron.2018.05.018. Neuron. 2018. PMID: 29953871 Free PMC article. Review.
-
Membrane trafficking and positioning of mGluRs at presynaptic and postsynaptic sites of excitatory synapses.Neuropharmacology. 2021 Dec 1;200:108799. doi: 10.1016/j.neuropharm.2021.108799. Epub 2021 Sep 28. Neuropharmacology. 2021. PMID: 34592242 Review.
-
Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder.Neurosci Biobehav Rev. 2017 Jun;77:14-31. doi: 10.1016/j.neubiorev.2017.02.024. Epub 2017 Feb 24. Neurosci Biobehav Rev. 2017. PMID: 28242339 Free PMC article. Review.
-
Structural biology of ionotropic glutamate delta receptors and their crosstalk with metabotropic glutamate receptors.Neuropharmacology. 2021 Sep 15;196:108683. doi: 10.1016/j.neuropharm.2021.108683. Epub 2021 Jun 26. Neuropharmacology. 2021. PMID: 34181979 Review.
-
Glutamate released spontaneously from astrocytes sets the threshold for synaptic plasticity.Eur J Neurosci. 2011 Apr;33(8):1483-92. doi: 10.1111/j.1460-9568.2011.07631.x. Epub 2011 Mar 14. Eur J Neurosci. 2011. PMID: 21395864
Cited by
-
Optical Assessment of Nociceptive TRP Channel Function at the Peripheral Nerve Terminal.Int J Mol Sci. 2021 Jan 6;22(2):481. doi: 10.3390/ijms22020481. Int J Mol Sci. 2021. PMID: 33418928 Free PMC article. Review.
-
SNAP-Tagged Nanobodies Enable Reversible Optical Control of a G Protein-Coupled Receptor via a Remotely Tethered Photoswitchable Ligand.ACS Chem Biol. 2018 Sep 21;13(9):2682-2688. doi: 10.1021/acschembio.8b00628. Epub 2018 Sep 7. ACS Chem Biol. 2018. PMID: 30141622 Free PMC article.
-
Optocontrol of glutamate receptor activity by single side-chain photoisomerization.Elife. 2017 May 23;6:e25808. doi: 10.7554/eLife.25808. Elife. 2017. PMID: 28534738 Free PMC article.
-
Optical Control of Dopamine Receptors Using a Photoswitchable Tethered Inverse Agonist.J Am Chem Soc. 2017 Dec 27;139(51):18522-18535. doi: 10.1021/jacs.7b07659. Epub 2017 Dec 13. J Am Chem Soc. 2017. PMID: 29166564 Free PMC article.
-
Optical control of AMPA receptors using a photoswitchable quinoxaline-2,3-dione antagonist.Chem Sci. 2017 Jan 1;8(1):611-615. doi: 10.1039/c6sc01621a. Epub 2016 Aug 24. Chem Sci. 2017. PMID: 28451208 Free PMC article.
References
-
- Callaway EM, Yuste R. Stimulating neurons with light. Curr Opi Neurobiol. 2002;12:587–592. - PubMed

