Genetic targeting of a small fluorescent zinc indicator to cell surface for monitoring zinc secretion
- PMID: 25572404
- PMCID: PMC4405262
- DOI: 10.1021/cb5007536
Genetic targeting of a small fluorescent zinc indicator to cell surface for monitoring zinc secretion
Abstract
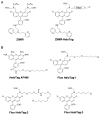
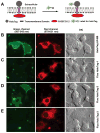
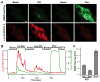
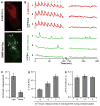
Numerous mammalian cells contain Zn2+ in their secretory granules. During secretion, Zn2+ is coreleased with granular cargos into extracellular medium so Zn2+ serves as a convenient surrogate marker for tracking the dynamics of secretion. Fluorescent Zn2+ sensors that can be selectively targeted to cells of interest would be invaluable tools for imaging Zn2+ release in multicellular systems including tissues and live animals. Exploiting the HaloTag labeling technology and using an optimized linker, we have engineered a fluorescent Zn2+ indicator that displayed a 15-fold fluorescence enhancement upon Zn2+ binding while reacting efficiently with a HaloTag enzyme in a cellular environment. Two-color imaging of ZIMIR-HaloTag and a red-emitting calcium indicator in pancreatic islet beta cells demonstrated that photoactivation of a channelrhodopsin was able to induce exocytosis of Zn2+/insulin granules and revealed heterogeneity in secretory activity along the cell membrane that was uncoupled from cellular Ca2+ activity. This integrated photonic approach for imaging and controlling the release of large dense core granules provides exquisite cellular selectivity and should facilitate future studies of stimulus-secretion coupling and paracrine signaling in secretory cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

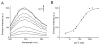
Similar articles
-
Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR).Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21063-8. doi: 10.1073/pnas.1109773109. Epub 2011 Dec 12. Proc Natl Acad Sci U S A. 2011. PMID: 22160693 Free PMC article.
-
In Vivo ZIMIR Imaging of Mouse Pancreatic Islet Cells Shows Oscillatory Insulin Secretion.Front Endocrinol (Lausanne). 2021 Mar 9;12:613964. doi: 10.3389/fendo.2021.613964. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33767668 Free PMC article.
-
Detection of secretion from single pancreatic beta-cells using extracellular fluorogenic reactions and confocal fluorescence microscopy.Anal Chem. 2000 Feb 15;72(4):711-7. doi: 10.1021/ac991085t. Anal Chem. 2000. PMID: 10701254
-
Fluorescent probes for monitoring regulated secretion.Curr Opin Chem Biol. 2013 Aug;17(4):672-81. doi: 10.1016/j.cbpa.2013.04.026. Epub 2013 May 24. Curr Opin Chem Biol. 2013. PMID: 23711436 Free PMC article. Review.
-
Zinc and insulin in pancreatic beta-cells.Endocrine. 2014 Mar;45(2):178-89. doi: 10.1007/s12020-013-0032-x. Epub 2013 Aug 24. Endocrine. 2014. PMID: 23979673 Review.
Cited by
-
ZIGIR, a Granule-Specific Zn2+ Indicator, Reveals Human Islet α Cell Heterogeneity.Cell Rep. 2020 Jul 14;32(2):107904. doi: 10.1016/j.celrep.2020.107904. Cell Rep. 2020. PMID: 32668245 Free PMC article.
-
Organelle-Level Labile Zn2+ Mapping Based on Targetable Fluorescent Sensors.ACS Sens. 2022 Mar 25;7(3):748-757. doi: 10.1021/acssensors.1c02153. Epub 2022 Mar 3. ACS Sens. 2022. PMID: 35238552 Free PMC article.
-
Mechanism-Based Strategy for Optimizing HaloTag Protein Labeling.JACS Au. 2022 May 18;2(6):1324-1337. doi: 10.1021/jacsau.2c00002. eCollection 2022 Jun 27. JACS Au. 2022. PMID: 35783171 Free PMC article.
-
Fine-tuned photochromic sulfonylureas for optical control of beta cell Ca2+ fluxes.Diabet Med. 2023 Dec;40(12):e15220. doi: 10.1111/dme.15220. Epub 2023 Sep 21. Diabet Med. 2023. PMID: 37669696 Free PMC article.
-
Probes for monitoring regulated exocytosis.Cell Calcium. 2017 Jun;64:65-71. doi: 10.1016/j.ceca.2017.01.002. Epub 2017 Jan 9. Cell Calcium. 2017. PMID: 28089267 Free PMC article. Review.
References
-
- Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. - PubMed
-
- Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. - PubMed
-
- Rutter GA, Varadi A, Tsuboi T, Parton L, Ravier M. Insulin secretion in health and disease: genomics, proteomics and single vesicle dynamics. Biochem Soc Trans. 2006;34:247–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

