Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages
- PMID: 25565375
- PMCID: PMC4286812
- DOI: 10.1038/ncomms6930
Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages
Abstract
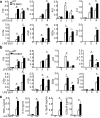
Signal transduction from toll-like receptors (TLRs) is important for innate immunity against infections, but deregulated TLR signalling contributes to inflammatory disorders. Here we show that myeloid cell-specific ablation of TRAF2 greatly promotes TLR-stimulated proinflammatory cytokine expression in macrophages and exacerbates colitis in an animal model of inflammatory bowel disease. TRAF2 deficiency does not enhance upstream signalling events, but it causes accumulation of two transcription factors, c-Rel and IRF5, known to mediate proinflammatory cytokine induction. Interestingly, TRAF2 controls the fate of c-Rel and IRF5 via a proteasome-dependent mechanism that also requires TRAF3 and the E3 ubiquitin ligase cIAP. We further show that TRAF2 also regulates inflammatory cytokine production in tumour-associated macrophages and facilitates tumour growth. These findings demonstrate an unexpected anti-inflammatory function of TRAF2 and suggest a proteasome-dependent mechanism that limits the proinflammatory TLR signalling.
Figures

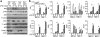




Similar articles
-
Protein tyrosine phosphatase SHP-1 positively regulates TLR-induced IL-12p40 production in macrophages through inhibition of phosphatidylinositol 3-kinase.J Leukoc Biol. 2010 May;87(5):845-55. doi: 10.1189/jlb.0409289. Epub 2010 Feb 9. J Leukoc Biol. 2010. PMID: 20145200
-
Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction.J Biol Chem. 2015 Apr 10;290(15):9660-73. doi: 10.1074/jbc.M114.609685. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716317 Free PMC article.
-
Nuclear presence of nuclear factor of activated T cells (NFAT) c3 and c4 is required for Toll-like receptor-activated innate inflammatory response of monocytes/macrophages.Cell Signal. 2011 Nov;23(11):1785-93. doi: 10.1016/j.cellsig.2011.06.013. Epub 2011 Jun 25. Cell Signal. 2011. PMID: 21726630 Free PMC article.
-
Targeting signaling factors for degradation, an emerging mechanism for TRAF functions.Immunol Rev. 2015 Jul;266(1):56-71. doi: 10.1111/imr.12311. Immunol Rev. 2015. PMID: 26085207 Free PMC article. Review.
-
[Toll-like receptor].Nihon Rinsho Meneki Gakkai Kaishi. 2005 Oct;28(5):309-17. doi: 10.2177/jsci.28.309. Nihon Rinsho Meneki Gakkai Kaishi. 2005. PMID: 16276044 Review. Japanese.
Cited by
-
Recent advances in understanding inhibitor of apoptosis proteins.F1000Res. 2018 Dec 3;7:F1000 Faculty Rev-1889. doi: 10.12688/f1000research.16439.1. eCollection 2018. F1000Res. 2018. PMID: 30631429 Free PMC article. Review.
-
Regulation of Interleukin-6 Receptor Signaling by TNF Receptor-Associated Factor 2 and 5 During Differentiation of Inflammatory CD4+ T Cells.Front Immunol. 2018 Aug 30;9:1986. doi: 10.3389/fimmu.2018.01986. eCollection 2018. Front Immunol. 2018. PMID: 30214449 Free PMC article. Review.
-
The impact of rumen-protected amino acids on the expression of key- genes involved in the innate immunity of dairy sheep.PLoS One. 2020 May 14;15(5):e0233192. doi: 10.1371/journal.pone.0233192. eCollection 2020. PLoS One. 2020. PMID: 32407360 Free PMC article.
-
Isoprenylcysteine Carboxyl Methyltransferase and Its Substrate Ras Are Critical Players Regulating TLR-Mediated Inflammatory Responses.Cells. 2020 May 14;9(5):1216. doi: 10.3390/cells9051216. Cells. 2020. PMID: 32422978 Free PMC article.
-
Targeting Notch-Activated M1 Macrophages Attenuates Joint Tissue Damage in a Mouse Model of Inflammatory Arthritis.J Bone Miner Res. 2017 Jul;32(7):1469-1480. doi: 10.1002/jbmr.3117. Epub 2017 Apr 10. J Bone Miner Res. 2017. PMID: 28256007 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

