Compound loss of function of nuclear receptors Tr2 and Tr4 leads to induction of murine embryonic β-type globin genes
- PMID: 25561507
- PMCID: PMC4342359
- DOI: 10.1182/blood-2014-10-605022
Compound loss of function of nuclear receptors Tr2 and Tr4 leads to induction of murine embryonic β-type globin genes
Abstract
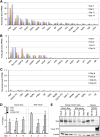
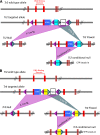
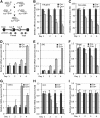
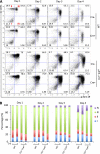
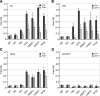
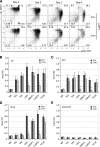
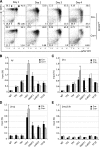
The orphan nuclear receptors TR2 and TR4 have been shown to play key roles in repressing the embryonic and fetal globin genes in erythroid cells. However, combined germline inactivation of Tr2 and Tr4 leads to periimplantation lethal demise in inbred mice. Hence, we have previously been unable to examine the consequences of their dual loss of function in adult definitive erythroid cells. To circumvent this issue, we generated conditional null mutants in both genes and performed gene inactivation in vitro in adult bone marrow cells. Compound Tr2/Tr4 loss of function led to induced expression of the embryonic εy and βh1 globins (murine counterparts of the human ε- and γ-globin genes). Additionally, TR2/TR4 function is required for terminal erythroid cell maturation. Loss of TR2/TR4 abolished their occupancy on the εy and βh1 gene promoters, and concurrently impaired co-occupancy by interacting corepressors. These data strongly support the hypothesis that the TR2/TR4 core complex is an adult stage-specific, gene-selective repressor of the embryonic globin genes. Detailed mechanistic understanding of the roles of TR2/TR4 and their cofactors in embryonic and fetal globin gene repression may ultimately enhance the discovery of novel therapeutic agents that can effectively inhibit their transcriptional activity and be safely applied to the treatment of β-globinopathies.
© 2015 by The American Society of Hematology.
Figures
Similar articles
-
Nuclear receptors TR2 and TR4 recruit multiple epigenetic transcriptional corepressors that associate specifically with the embryonic β-type globin promoters in differentiated adult erythroid cells.Mol Cell Biol. 2011 Aug;31(16):3298-311. doi: 10.1128/MCB.05310-11. Epub 2011 Jun 13. Mol Cell Biol. 2011. PMID: 21670149 Free PMC article.
-
Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4.EMBO J. 2007 May 2;26(9):2295-306. doi: 10.1038/sj.emboj.7601676. Epub 2007 Apr 12. EMBO J. 2007. PMID: 17431400 Free PMC article.
-
An embryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer.EMBO J. 2002 Jul 1;21(13):3434-42. doi: 10.1093/emboj/cdf340. EMBO J. 2002. PMID: 12093744 Free PMC article.
-
Roles of Nuclear Orphan Receptors TR2 and TR4 during Hematopoiesis.Genes (Basel). 2024 Apr 27;15(5):563. doi: 10.3390/genes15050563. Genes (Basel). 2024. PMID: 38790192 Free PMC article. Review.
-
Recent advances in the TR2 and TR4 orphan receptors of the nuclear receptor superfamily.J Steroid Biochem Mol Biol. 2002 Aug;81(4-5):291-308. doi: 10.1016/s0960-0760(02)00118-8. J Steroid Biochem Mol Biol. 2002. PMID: 12361719 Review.
Cited by
-
Fetal hemoglobin in sickle cell anemia: The Arab-Indian haplotype and new therapeutic agents.Am J Hematol. 2017 Nov;92(11):1233-1242. doi: 10.1002/ajh.24872. Epub 2017 Aug 17. Am J Hematol. 2017. PMID: 28736939 Free PMC article. Review.
-
Physiological and Aberrant γ-Globin Transcription During Development.Front Cell Dev Biol. 2021 Apr 1;9:640060. doi: 10.3389/fcell.2021.640060. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869190 Free PMC article. Review.
-
The Coup-TFII orphan nuclear receptor is an activator of the γ-globin gene.Haematologica. 2021 Feb 1;106(2):474-482. doi: 10.3324/haematol.2019.241224. Haematologica. 2021. PMID: 32107331 Free PMC article.
-
Mouse Models of Erythropoiesis and Associated Diseases.Methods Mol Biol. 2018;1698:37-65. doi: 10.1007/978-1-4939-7428-3_3. Methods Mol Biol. 2018. PMID: 29076083 Free PMC article. Review.
-
Heterochromatin Protein 1γ Is a Novel Epigenetic Repressor of Human Embryonic ϵ-Globin Gene Expression.J Biol Chem. 2017 Mar 24;292(12):4811-4817. doi: 10.1074/jbc.M116.768515. Epub 2017 Feb 1. J Biol Chem. 2017. PMID: 28154185 Free PMC article.
References
-
- Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373–383. - PubMed
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. - PubMed
-
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol. 1995;2(2):103–108. - PubMed
-
- Sunshine HR, Hofrichter J, Eaton WA. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978;275(5677):238–240. - PubMed
-
- Stamatoyannopoulos JA, Nienhuis AW. Therapeutic approaches to hemoglobin switching in treatment of hemoglobinopathies. Annu Rev Med. 1992;43:497–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

