αvβ6 integrin is required for TGFβ1-mediated matrix metalloproteinase2 expression
- PMID: 25558779
- PMCID: PMC4363118
- DOI: 10.1042/BJ20140698
αvβ6 integrin is required for TGFβ1-mediated matrix metalloproteinase2 expression
Abstract
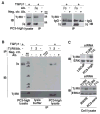
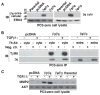
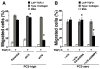
Transforming growth factor (TGF) β1 activity depends on a complex signalling cascade that controls expression of several genes. Among others, TGFβ1 regulates expression of matrix metalloproteinases (MMPs) through activation of Smads. In the present study, we demonstrate for the first time that the αvβ6 integrin interacts with TGFβ receptor II (TβRII) through the β6 cytoplasmic domain and promotes Smad3 activation in prostate cancer (PrCa) cells. Another related αv integrin, αvβ5, as well as the αvβ6/3 integrin, which contains a chimeric form of β6 with a β3 cytoplasmic domain, do not associate with TβRII and fail to show similar responses. We provide evidence that αvβ6 is required for up-regulation of MMP2 by TGFβ1 through a Smad3-mediated transcriptional programme in PrCa cells. The functional relevance of these results is underscored by the finding that αvβ6 modulates cell migration in an MMP2-dependent manner on an αvβ6-specific ligand, latency-associated peptide (LAP)-TGFβ. Overall, these mechanistic studies establish that expression of a single integrin, αvβ6, is sufficient to promote activation of Smad3, regulation of MMP2 levels and consequent catalytic activity, as well as cell migration. Our study describes a new TGFβ1-αvβ6-MMP2 signalling pathway that, given TGFβ1 pro-metastatic activity, may have profound implications for PrCa therapy.
Conflict of interest statement
The other authors do not have any conflict of interests.
Figures





Similar articles
-
The role of integrins in TGFβ activation in the tumour stroma.Cell Tissue Res. 2016 Sep;365(3):657-73. doi: 10.1007/s00441-016-2474-y. Epub 2016 Aug 12. Cell Tissue Res. 2016. PMID: 27515461 Free PMC article. Review.
-
Integrin αvβ6 promotes an osteolytic program in cancer cells by upregulating MMP2.Cancer Res. 2014 Mar 1;74(5):1598-608. doi: 10.1158/0008-5472.CAN-13-1796. Epub 2014 Jan 2. Cancer Res. 2014. PMID: 24385215 Free PMC article.
-
Amplification of TGFβ Induced ITGB6 Gene Transcription May Promote Pulmonary Fibrosis.PLoS One. 2016 Aug 5;11(8):e0158047. doi: 10.1371/journal.pone.0158047. eCollection 2016. PLoS One. 2016. PMID: 27494713 Free PMC article.
-
TGFbeta1 signaling via alphaVbeta6 integrin.Mol Cancer. 2003 Aug 7;2:28. doi: 10.1186/1476-4598-2-28. Mol Cancer. 2003. Retraction in: Mol Cancer. 2004 Jan 14;3:2. doi: 10.1186/1476-4598-3-2 PMID: 12935295 Free PMC article. Retracted.
-
Defining the role of integrin alphavbeta6 in cancer.Curr Drug Targets. 2009 Jul;10(7):645-52. doi: 10.2174/138945009788680374. Curr Drug Targets. 2009. PMID: 19601768 Free PMC article. Review.
Cited by
-
αvβ6 Integrin Promotes Castrate-Resistant Prostate Cancer through JNK1-Mediated Activation of Androgen Receptor.Cancer Res. 2016 Sep 1;76(17):5163-74. doi: 10.1158/0008-5472.CAN-16-0543. Epub 2016 Jul 22. Cancer Res. 2016. PMID: 27450452 Free PMC article.
-
Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells.J Clin Invest. 2021 Jul 15;131(14):e142116. doi: 10.1172/JCI142116. J Clin Invest. 2021. PMID: 34138753 Free PMC article.
-
Disparate Interferon Signaling and Shared Aberrant Basaloid Cells in Single-Cell Profiling of Idiopathic Pulmonary Fibrosis and Systemic Sclerosis-Associated Interstitial Lung Disease.Front Immunol. 2021 Mar 30;12:595811. doi: 10.3389/fimmu.2021.595811. eCollection 2021. Front Immunol. 2021. PMID: 33859634 Free PMC article.
-
The αvβ6 integrin in cancer cell-derived small extracellular vesicles enhances angiogenesis.J Extracell Vesicles. 2020 May 20;9(1):1763594. doi: 10.1080/20013078.2020.1763594. eCollection 2020. J Extracell Vesicles. 2020. PMID: 32595914 Free PMC article.
-
The role of integrins in TGFβ activation in the tumour stroma.Cell Tissue Res. 2016 Sep;365(3):657-73. doi: 10.1007/s00441-016-2474-y. Epub 2016 Aug 12. Cell Tissue Res. 2016. PMID: 27515461 Free PMC article. Review.
References
-
- Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. - PubMed
-
- Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, Otey CA, Zhau HE, Chung LW. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001;12:99–107. - PubMed
-
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

