Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine
- PMID: 25549971
- PMCID: PMC4369168
- DOI: 10.1007/s00401-014-1380-1
Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine
Abstract
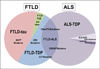
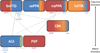
Frontotemporal lobar degeneration (FTLD) comprises two main classes of neurodegenerative diseases characterized by neuronal/glial proteinaceous inclusions (i.e., proteinopathies) including tauopathies (i.e., FTLD-Tau) and TDP-43 proteinopathies (i.e., FTLD-TDP) while other very rare forms of FTLD are known such as FTLD with FUS pathology (FTLD-FUS). This review focuses mainly on FTLD-Tau and FLTD-TDP, which may present as several clinical syndromes: a behavioral/dysexecutive syndrome (behavioral variant frontotemporal dementia); language disorders (primary progressive aphasia variants); and motor disorders (amyotrophic lateral sclerosis, corticobasal syndrome, progressive supranuclear palsy syndrome). There is considerable heterogeneity in clinical presentations of underlying neuropathology and current clinical criteria do not reliably predict underlying proteinopathies ante-mortem. In contrast, molecular etiologies of hereditary FTLD are consistently associated with specific proteinopathies. These include MAPT mutations with FTLD-Tau and GRN, C9orf72, VCP and TARDBP with FTLD-TDP. The last decade has seen a rapid expansion in our knowledge of the molecular pathologies associated with this clinically and neuropathologically heterogeneous group of FTLD diseases. Moreover, in view of current limitations to reliably diagnose specific FTLD neuropathologies prior to autopsy, we summarize the current state of the science in FTLD biomarker research including neuroimaging, biofluid and genetic analyses. We propose that combining several of these biomarker modalities will improve diagnostic specificity in FTLD through a personalized medicine approach. The goals of these efforts are to enhance power for clinical trials focused on slowing or preventing progression of spread of tau, TDP-43 and other FTLD-associated pathologies and work toward the goal of defining clinical endophenotypes of FTD.
Figures



Similar articles
-
Frontotemporal lobar degeneration: Pathogenesis, pathology and pathways to phenotype.Brain Pathol. 2017 Nov;27(6):723-736. doi: 10.1111/bpa.12486. Epub 2017 Mar 2. Brain Pathol. 2017. PMID: 28100023 Free PMC article. Review.
-
Frontotemporal lobar degeneration: diversity of FTLD lesions.Rev Neurol (Paris). 2013 Oct;169(10):786-92. doi: 10.1016/j.neurol.2013.07.015. Epub 2013 Sep 12. Rev Neurol (Paris). 2013. PMID: 24035575 Review.
-
No interaction between tau and TDP-43 pathologies in either frontotemporal lobar degeneration or motor neurone disease.Neuropathol Appl Neurobiol. 2014 Dec;40(7):844-54. doi: 10.1111/nan.12155. Neuropathol Appl Neurobiol. 2014. PMID: 24861427
-
Frontotemporal dementia: implications for understanding Alzheimer disease.Cold Spring Harb Perspect Med. 2012 Feb;2(2):a006254. doi: 10.1101/cshperspect.a006254. Cold Spring Harb Perspect Med. 2012. PMID: 22355793 Free PMC article. Review.
-
[Pathomechanisms and clinical aspects of frontotemporal lobar degeneration].Nervenarzt. 2017 Feb;88(2):163-172. doi: 10.1007/s00115-016-0259-x. Nervenarzt. 2017. PMID: 27999880 German.
Cited by
-
Interhemispheric connectivity in amyotrophic lateral sclerosis: A near-infrared spectroscopy and diffusion tensor imaging study.Neuroimage Clin. 2016 Sep 29;12:666-672. doi: 10.1016/j.nicl.2016.09.020. eCollection 2016. Neuroimage Clin. 2016. PMID: 27761397 Free PMC article.
-
Machine Learning Approach Predicts Probability of Time to Stage-Specific Conversion of Alzheimer's Disease.J Alzheimers Dis. 2022;90(2):891-903. doi: 10.3233/JAD-220590. J Alzheimers Dis. 2022. PMID: 36189595 Free PMC article.
-
Defining and predicting transdiagnostic categories of neurodegenerative disease.Nat Biomed Eng. 2020 Aug;4(8):787-800. doi: 10.1038/s41551-020-0593-y. Epub 2020 Aug 3. Nat Biomed Eng. 2020. PMID: 32747831 Free PMC article.
-
Tau physiology and pathomechanisms in frontotemporal lobar degeneration.J Neurochem. 2016 Aug;138 Suppl 1(Suppl Suppl 1):71-94. doi: 10.1111/jnc.13600. Epub 2016 Jun 15. J Neurochem. 2016. PMID: 27306859 Free PMC article. Review.
-
Microglial activation in the frontal cortex predicts cognitive decline in frontotemporal dementia.Brain. 2023 Aug 1;146(8):3221-3231. doi: 10.1093/brain/awad078. Brain. 2023. PMID: 36883644 Free PMC article.
References
-
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobagyi T, Shaw CE. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta neuropathologica. 2011;122:691–702. - PubMed
-
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer's disease. Brain : a journal of neurology. 2007;130:2636–2645. - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochemical and biophysical research communications. 2006;351:602–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG032953/AG/NIA NIH HHS/United States
- P01-AG03991/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- P01-AG17586/AG/NIA NIH HHS/United States
- K08 AG039510/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- NS088341/NS/NINDS NIH HHS/United States
- P50-AG05681/AG/NIA NIH HHS/United States
- P01-AG032953/AG/NIA NIH HHS/United States
- P30-AG10124/AG/NIA NIH HHS/United States
- K23 NS088341/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

