Quality assessment and optimization of purified protein samples: why and how?
- PMID: 25547134
- PMCID: PMC4299812
- DOI: 10.1186/s12934-014-0180-6
Quality assessment and optimization of purified protein samples: why and how?
Abstract
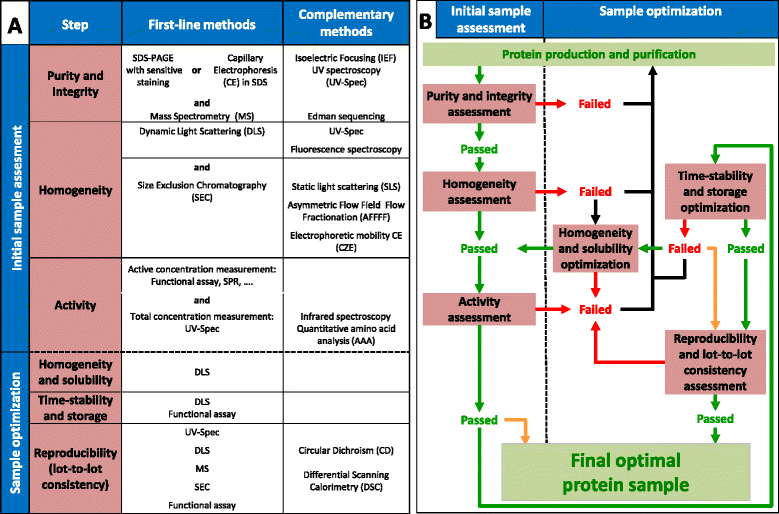
Purified protein quality control is the final and critical check-point of any protein production process. Unfortunately, it is too often overlooked and performed hastily, resulting in irreproducible and misleading observations in downstream applications. In this review, we aim at proposing a simple-to-follow workflow based on an ensemble of widely available physico-chemical technologies, to assess sequentially the essential properties of any protein sample: purity and integrity, homogeneity and activity. Approaches are then suggested to optimize the homogeneity, time-stability and storage conditions of purified protein preparations, as well as methods to rapidly evaluate their reproducibility and lot-to-lot consistency.
Figures
Similar articles
-
Guidelines to reach high-quality purified recombinant proteins.Appl Microbiol Biotechnol. 2018 Jan;102(1):81-92. doi: 10.1007/s00253-017-8623-8. Epub 2017 Nov 18. Appl Microbiol Biotechnol. 2018. PMID: 29151158 Review.
-
Production, purification, and chemical stability of recombinant human interferon-γ in low oxygen tension condition: a formulation approach.Prep Biochem Biotechnol. 2013;43(6):586-600. doi: 10.1080/10826068.2012.762716. Prep Biochem Biotechnol. 2013. PMID: 23742090
-
Assessing and Improving Protein Sample Quality.Methods Mol Biol. 2021;2263:3-46. doi: 10.1007/978-1-0716-1197-5_1. Methods Mol Biol. 2021. PMID: 33877592
-
Crystallization of Recombinant α-Actinin and Related Proteins.Methods Mol Biol. 2018;1721:95-103. doi: 10.1007/978-1-4939-7546-4_9. Methods Mol Biol. 2018. PMID: 29423850 Review.
-
Cloning and optimization of induction conditions for mature PsaA (pneumococcal surface adhesin A) expression in Escherichia coli and recombinant protein stability during long-term storage.Protein Expr Purif. 2011 Jul;78(1):38-47. doi: 10.1016/j.pep.2011.02.013. Epub 2011 Mar 6. Protein Expr Purif. 2011. PMID: 21362478
Cited by
-
Membranes under the Magnetic Lens: A Dive into the Diverse World of Membrane Protein Structures Using Cryo-EM.Chem Rev. 2022 Sep 14;122(17):13989-14017. doi: 10.1021/acs.chemrev.1c00837. Epub 2022 Jul 18. Chem Rev. 2022. PMID: 35849490 Free PMC article. Review.
-
Glucagon-like peptide 1 aggregates into low-molecular-weight oligomers off-pathway to fibrillation.Biophys J. 2023 Jun 20;122(12):2475-2488. doi: 10.1016/j.bpj.2023.04.027. Epub 2023 May 2. Biophys J. 2023. PMID: 37138517 Free PMC article.
-
Engineered AAA+ proteases reveal principles of proteolysis at the mitochondrial inner membrane.Nat Commun. 2016 Oct 27;7:13301. doi: 10.1038/ncomms13301. Nat Commun. 2016. PMID: 27786171 Free PMC article.
-
Basic regulatory science behind drug substance and drug product specifications of monoclonal antibodies and other protein therapeutics.J Pharm Anal. 2024 Jun;14(6):100916. doi: 10.1016/j.jpha.2023.12.006. Epub 2023 Dec 10. J Pharm Anal. 2024. PMID: 39035218 Free PMC article. Review.
-
Measuring Protein Aggregation and Stability Using High-Throughput Biophysical Approaches.Front Mol Biosci. 2022 May 16;9:890862. doi: 10.3389/fmolb.2022.890862. eCollection 2022. Front Mol Biosci. 2022. PMID: 35651816 Free PMC article.
References
-
- Gräslund S, Nordlund P, Weigelt J, Hallberg BM, Bray J, Gileadi O, Knapp S, Oppermann U, Arrowsmith C, Hui R, Ming J, Dhe-Paganon S, Park H, Savchenko A, Yee A, Edwards A, Vincentelli R, Cambillau C, Kim R, Kim S-H, Rao Z, Shi Y, Terwilliger TC, Kim C-Y, Hung L-W, Waldo GS, Peleg Y, Albeck S, Unger T, Dym O, et al. Protein production and purification. Nat Methods. 2008;5:135–146. doi: 10.1038/nmeth.f.202. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

