Conditional knockout of prolyl hydroxylase domain protein 2 attenuates high fat-diet-induced cardiac dysfunction in mice
- PMID: 25546437
- PMCID: PMC4278833
- DOI: 10.1371/journal.pone.0115974
Conditional knockout of prolyl hydroxylase domain protein 2 attenuates high fat-diet-induced cardiac dysfunction in mice
Abstract
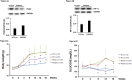
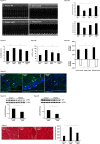
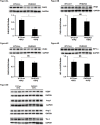
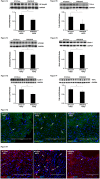
Oxygen sensor prolyl hydroxylases (PHDs) play important roles in the regulation of HIF-α and cell metabolisms. This study was designed to investigate the direct role of PHD2 in high fat-diet (HFD)-induced cardiac dysfunction. In HFD fed mice, PHD2 expression was increased without significant changes in PHD1 and PHD3 levels in the heart. This was accompanied by a significant upregulation of myeloid differentiation factor 88 (MYD88) and NF-κB. To explore the role of PHD2 in HFD-induced cardiac dysfunction, PHD2 conditional knockout mice were fed a HFD for 16 weeks. Intriguingly, knockout of PHD2 significantly reduced MYD88 and NF-κb expression in HFD mouse hearts. Moreover, knockout of PHD2 inhibited TNFα and ICAM-1 expression, and reduced cell apoptosis and macrophage infiltration in HFD mice. This was accompanied by a significant improvement of cardiac function. Most importantly, conditional knockout of PHD2 at late stage in HFD mice significantly improved glucose tolerance and reversed cardiac dysfunction. Our studies demonstrate that PHD2 activity is a critical contributor to the HFD-induced cardiac dysfunction. Inhibition of PHD2 attenuates HFD-induced cardiac dysfunction by a mechanism involving suppression of MYD88/NF-κb pathway and inflammation.
Conflict of interest statement
Figures



Similar articles
-
Regulatory Role of Endothelial PHD2 in the Hepatic Steatosis.Cell Physiol Biochem. 2018;48(3):1003-1011. doi: 10.1159/000491968. Epub 2018 Jul 23. Cell Physiol Biochem. 2018. PMID: 30036883 Free PMC article.
-
Macrophage HIF-1α mediates obesity-related adipose tissue dysfunction via interleukin-1 receptor-associated kinase M.Am J Physiol Endocrinol Metab. 2020 May 1;318(5):E689-E700. doi: 10.1152/ajpendo.00174.2019. Epub 2020 Mar 10. Am J Physiol Endocrinol Metab. 2020. PMID: 32154744 Free PMC article.
-
Obesity-induced kidney injury is attenuated by amelioration of aberrant PHD2 activation in proximal tubules.Sci Rep. 2016 Nov 9;6:36533. doi: 10.1038/srep36533. Sci Rep. 2016. PMID: 27827416 Free PMC article.
-
Prolyl hydroxylase domain 2 deficiency promotes skeletal muscle fiber-type transition via a calcineurin/NFATc1-dependent pathway.Skelet Muscle. 2016 Mar 5;6:5. doi: 10.1186/s13395-016-0079-5. eCollection 2016. Skelet Muscle. 2016. PMID: 26949511 Free PMC article.
-
The roles and signaling pathways of prolyl-4-hydroxylase 2 in the tumor microenvironment.Chem Biol Interact. 2019 Apr 25;303:40-49. doi: 10.1016/j.cbi.2019.02.019. Epub 2019 Feb 25. Chem Biol Interact. 2019. PMID: 30817904 Review.
Cited by
-
A Sex-Specific Role of Endothelial Sirtuin 3 on Blood Pressure and Diastolic Dysfunction in Female Mice.Int J Mol Sci. 2020 Dec 21;21(24):9744. doi: 10.3390/ijms21249744. Int J Mol Sci. 2020. PMID: 33371209 Free PMC article.
-
LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2α/Notch3 pathways.Sci Rep. 2016 Feb 12;6:20931. doi: 10.1038/srep20931. Sci Rep. 2016. PMID: 26868537 Free PMC article.
-
The role of α-ketoglutarate and the hypoxia sensing pathway in the regulation of pancreatic β-cell function.Islets. 2020 Sep 2;12(5):108-119. doi: 10.1080/19382014.2020.1802183. Epub 2020 Sep 2. Islets. 2020. PMID: 32876527 Free PMC article. Review.
-
Hypoxia inducible factor-1 alpha and prolinhydroxlase 2 polymorphisms in patients with severe sepsis: a prospective observational trial.BMC Anesthesiol. 2016 Aug 11;16(1):61. doi: 10.1186/s12871-016-0225-y. BMC Anesthesiol. 2016. PMID: 27515179 Free PMC article.
-
Isoform-specific Roles of Prolyl Hydroxylases in the Regulation of Pancreatic β-Cell Function.Endocrinology. 2022 Jan 1;163(1):bqab226. doi: 10.1210/endocr/bqab226. Endocrinology. 2022. PMID: 34718519 Free PMC article.
References
-
- Nwogu JI, Geenen D, Bean M, Brenner MC, Huang X, et al. (2001) Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation 104:2216–2221 doi:10.1161/hc4301.097193. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

