The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding
- PMID: 25544774
- PMCID: PMC4385857
- DOI: 10.18632/oncotarget.2958
The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding
Abstract
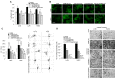
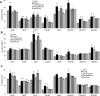
Tspan8 and CD151 are metastasis-promoting tetraspanins and a knockdown (kd) of Tspan8 or CD151 and most pronounced of both tetraspanins affects the metastatic potential of the rat pancreatic adenocarcinoma line ASML. Approaching to elaborate the underlying mechanism, we compared ASMLwt, -CD151kd and/or Tspan8kd clones. We focused on tumor exosomes, as exosomes play a major role in tumor progression and tetraspanins are suggested to be engaged in exosome targeting. ASML-CD151/Tspan8kd cells poorly metastasize, but regain metastatic capacity, when rats are pretreated with ASMLwt, but not ASML-CD151kd and/or -Tspan8kd exosomes. Both exosomal CD151 and Tspan8 contribute to host matrix remodelling due to exosomal tetraspanin-integrin and tetraspanin-protease associations. ASMLwt exosomes also support stroma cell activation with upregulation of cytokines, cytokine receptors and proteases and promote inflammatory cytokine expression in hematopoietic cells. Finally, CD151-/Tspan8-competent exosomes support EMT gene expression in poorly-metastatic ASML-CD151/Tspan8kd cells. These effects are not seen or are weakened using ASML-CD151kd or -Tspan8kd exosomes, which is at least partly due to reduced binding/uptake of CD151- and/or Tspan8-deficient exosomes. Thus, CD151- and Tspan8-competent tumor exosomes support matrix degradation, reprogram stroma and hematopoietic cells and drive non-metastatic ASML-CD151/Tspan8kd cells towards a motile phenotype.
Conflict of interest statement
The authors declare that they have no competing interests
Figures



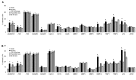
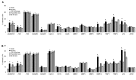



Similar articles
-
Tspan8 and CD151 promote metastasis by distinct mechanisms.Eur J Cancer. 2013 Sep;49(13):2934-48. doi: 10.1016/j.ejca.2013.03.032. Epub 2013 May 14. Eur J Cancer. 2013. PMID: 23683890
-
Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression.J Exp Clin Cancer Res. 2018 Dec 12;37(1):312. doi: 10.1186/s13046-018-0961-6. J Exp Clin Cancer Res. 2018. Retraction in: J Exp Clin Cancer Res. 2025 Jan 16;44(1):16. doi: 10.1186/s13046-025-03284-z PMID: 30541597 Free PMC article. Retracted.
-
Distorted leukocyte migration, angiogenesis, wound repair and metastasis in Tspan8 and Tspan8/CD151 double knockout mice indicate complementary activities of Tspan8 and CD51.Biochim Biophys Acta Mol Cell Res. 2018 Feb;1865(2):379-391. doi: 10.1016/j.bbamcr.2017.11.007. Epub 2017 Nov 11. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29138006
-
Joint features and complementarities of Tspan8 and CD151 revealed in knockdown and knockout models.Biochem Soc Trans. 2017 Apr 15;45(2):437-447. doi: 10.1042/BST20160298. Biochem Soc Trans. 2017. PMID: 28408484 Review.
-
[The role of exosomal tetraspanins and proteases in tumor progression].Biomed Khim. 2018 Mar;64(2):123-133. doi: 10.18097/PBMC20186402123. Biomed Khim. 2018. PMID: 29723143 Review. Russian.
Cited by
-
Molecular Regulation and Oncogenic Functions of TSPAN8.Cells. 2024 Jan 19;13(2):193. doi: 10.3390/cells13020193. Cells. 2024. PMID: 38275818 Free PMC article. Review.
-
The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses.Front Immunol. 2020 May 15;11:940. doi: 10.3389/fimmu.2020.00940. eCollection 2020. Front Immunol. 2020. PMID: 32499786 Free PMC article. Review.
-
Expression of tetraspanins NET-6 and CD151 in breast cancer as a potential tumor biomarker.Clin Exp Med. 2019 Aug;19(3):377-384. doi: 10.1007/s10238-019-00554-x. Epub 2019 Apr 20. Clin Exp Med. 2019. PMID: 31004251
-
Identification of prostate cancer biomarkers in urinary exosomes.Oncotarget. 2015 Oct 6;6(30):30357-76. doi: 10.18632/oncotarget.4851. Oncotarget. 2015. PMID: 26196085 Free PMC article.
-
Tumor-derived extracellular vesicles modulate innate immune responses to affect tumor progression.Front Immunol. 2022 Nov 2;13:1045624. doi: 10.3389/fimmu.2022.1045624. eCollection 2022. Front Immunol. 2022. PMID: 36405712 Free PMC article. Review.
References
-
- Sales KM, Winslet MC, Seifalian AM. Stem cells and cancer: an overview. Stem Cell Rev. 2007;3:249–255. - PubMed
-
- Chen SY, Huang YC, Liu SP, Tsai FJ, Shyu WC, Lin SZ. An overview of concepts for cancer stem cells. Cell Transplant. 2011;20:113–120. - PubMed
-
- Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. - PubMed
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

