Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice
- PMID: 25544360
- PMCID: PMC4326786
- DOI: 10.1038/aps.2014.112
Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice
Abstract
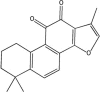
Aim: To study the effects of tanshinone IIA (TIIA) on lipopolysaccharide (LPS)-induced acute lung injury in mice and the underlying mechanisms.
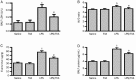
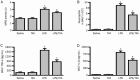
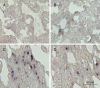
Methods: Mice were injected with LPS (10 mg/kg, i.p.), then treated with TIIA (10 mg/kg, i.p.). Seven hours after LPS injection, the lungs were collected for histological study. Protein, LDH, TNF-α and IL-1β levels in bronchoalveolar lavage fluid (BALF) and myeloperoxidase (MPO) activity in lungs were measured. Cell apoptosis and Bcl-2, caspase-3, NF-κB and HIF-1α expression in lungs were assayed.
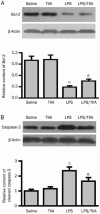
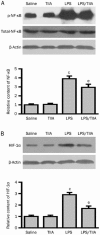
Results: LPS caused marked histological changes in lungs, accompanied by significantly increased lung W/D ratio, protein content and LDH level in BALF, and Evans blue leakage. LPS markedly increased neutrophil infiltration in lungs and inflammatory cytokines in BALF. Furthermore, LPS induced cell apoptosis in lungs, as evidenced by increased TUNEL-positive cells, decreased Bcl-2 content and increased cleaved caspase-3 content. Moreover, LPS significantly increased the expression of NF-κB and HIF-1α in lungs. Treatment of LPS-injected mice with TIIA significantly alleviated these pathological changes in lungs.
Conclusion: TIIA alleviates LPS-induced acute lung injury in mice by suppressing inflammatory responses and apoptosis, which is mediated via inhibition of the NF-κB and HIF-1α pathways.
Figures

Similar articles
-
Protectin D1 promotes resolution of inflammation in a murine model of lipopolysaccharide-induced acute lung injury via enhancing neutrophil apoptosis.Chin Med J (Engl). 2014;127(5):810-4. Chin Med J (Engl). 2014. PMID: 24571867
-
Tanshinone IIA-induced attenuation of lung injury in endotoxemic mice is associated with reduction of hypoxia-inducible factor 1α expression.Am J Respir Cell Mol Biol. 2011 Nov;45(5):1028-35. doi: 10.1165/rcmb.2011-0113OC. Epub 2011 May 26. Am J Respir Cell Mol Biol. 2011. PMID: 21622293
-
Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice.Chin J Nat Med. 2014 Nov;12(11):841-6. doi: 10.1016/S1875-5364(14)60126-6. Chin J Nat Med. 2014. PMID: 25480515
-
Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro.Int Immunopharmacol. 2015 Jul;27(1):138-47. doi: 10.1016/j.intimp.2015.05.005. Epub 2015 May 14. Int Immunopharmacol. 2015. PMID: 25981110
-
Tanshinone IIA reduces lethality and acute lung injury in LPS-treated mice by inhibition of PLA2 activity.Eur J Pharmacol. 2009 Apr 1;607(1-3):194-200. doi: 10.1016/j.ejphar.2009.02.003. Eur J Pharmacol. 2009. PMID: 19326571
Cited by
-
Rescue therapy with Tanshinone IIA hinders transition of acute kidney injury to chronic kidney disease via targeting GSK3β.Sci Rep. 2016 Nov 18;6:36698. doi: 10.1038/srep36698. Sci Rep. 2016. PMID: 27857162 Free PMC article.
-
The anti-inflammatory activities of ethanol extract from Dan-Lou prescription in vivo and in vitro.BMC Complement Altern Med. 2015 Sep 9;15:317. doi: 10.1186/s12906-015-0848-4. BMC Complement Altern Med. 2015. PMID: 26354089 Free PMC article.
-
Protective effects of hesperetin on lipopolysaccharide-induced acute lung injury in a rat model.Turk Gogus Kalp Damar Cerrahisi Derg. 2020 Apr 22;28(2):359-368. doi: 10.5606/tgkdc.dergisi.2020.18816. eCollection 2019 Jun. Turk Gogus Kalp Damar Cerrahisi Derg. 2020. PMID: 32551168 Free PMC article.
-
Astringin protects LPS-induced toxicity by suppressing oxidative stress and inflammation via suppression of PI3K/AKT/NF-κB pathway for pediatric acute lung injury.Naunyn Schmiedebergs Arch Pharmacol. 2023 Oct;396(10):2369-2377. doi: 10.1007/s00210-023-02439-z. Epub 2023 May 17. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37193771
-
Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms.Pharmacol Res. 2021 Jan;163:105224. doi: 10.1016/j.phrs.2020.105224. Epub 2020 Sep 29. Pharmacol Res. 2021. PMID: 33007416 Free PMC article. Review.
References
-
- Touqui L, Arbibe L. A role for phospholipase A2 in ARDS pathogenesis. Mol Med Today. 1999;5:244–9. - PubMed
-
- Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155:1187–205. - PubMed
-
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. - PubMed
-
- Downey GP, Dong Q, Kruger J, Dedhar S, Cherapanov V. Regulation of neutrophil activation in acute lung injury. Chest. 1999;116:46S–54S. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

