Experimental human infection with Norwalk virus elicits a surrogate neutralizing antibody response with cross-genogroup activity
- PMID: 25540269
- PMCID: PMC4308873
- DOI: 10.1128/CVI.00516-14
Experimental human infection with Norwalk virus elicits a surrogate neutralizing antibody response with cross-genogroup activity
Abstract
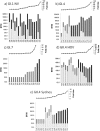
The human noroviruses (NoVs) are genetically diverse, rapidly evolving RNA viruses and are the major cause of epidemic gastroenteritis of humans. Serum antibodies that block the interaction of NoVs and NoV viruslike particles (VLPs) with host attachment factors are considered surrogate neutralizing antibodies in the absence of cell culture and small-animal replication models for the human NoVs. A serological assay for NoV-blocking antibodies was used to assess the breadth of the heterotypic antibody response in the context of an experimental challenge study with a human NoV. Heterotypic histo-blood group antigen (HBGA)-blocking activity against GI.4, GI.7, and GII.4 NoVs increased significantly in the serum of individuals (n = 18) infected with Norwalk virus (GI.1). Although the fold increases and peak titers of heterotypic antibody were more modest than titers of antibody reactive with the challenge antigen, Norwalk virus infection elicited a serological rise even against the novel Sydney variant of GII.4 NoVs. These observations indicate that the development of a broadly cross-protective NoV vaccine containing a limited number of genotypes may be possible.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Heterotypic humoral and cellular immune responses following Norwalk virus infection.J Virol. 2010 Feb;84(4):1800-15. doi: 10.1128/JVI.02179-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007270 Free PMC article.
-
Genotype considerations for virus-like particle-based bivalent norovirus vaccine composition.Clin Vaccine Immunol. 2015 Jun;22(6):656-63. doi: 10.1128/CVI.00015-15. Epub 2015 Apr 22. Clin Vaccine Immunol. 2015. PMID: 25903355 Free PMC article.
-
Functionality and avidity of norovirus-specific antibodies and T cells induced by GII.4 virus-like particles alone or co-administered with different genotypes.Vaccine. 2018 Jan 25;36(4):484-490. doi: 10.1016/j.vaccine.2017.12.009. Epub 2017 Dec 13. Vaccine. 2018. PMID: 29246474
-
Norovirus and histo-blood group antigens.Jpn J Infect Dis. 2011;64(2):95-103. Jpn J Infect Dis. 2011. PMID: 21519121 Review.
-
Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis.Expert Rev Vaccines. 2013 Feb;12(2):155-67. doi: 10.1586/erv.12.145. Expert Rev Vaccines. 2013. PMID: 23414407 Review.
Cited by
-
Structural basis for norovirus neutralization by an HBGA blocking human IgA antibody.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):E5830-E5837. doi: 10.1073/pnas.1609990113. Epub 2016 Sep 19. Proc Natl Acad Sci U S A. 2016. PMID: 27647885 Free PMC article.
-
Correlates of Protection against Norovirus Infection and Disease-Where Are We Now, Where Do We Go?PLoS Pathog. 2016 Apr 26;12(4):e1005334. doi: 10.1371/journal.ppat.1005334. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27115358 Free PMC article. Review. No abstract available.
-
Viral gastroenteritis.Lancet. 2018 Jul 14;392(10142):175-186. doi: 10.1016/S0140-6736(18)31128-0. Epub 2018 Jun 29. Lancet. 2018. PMID: 30025810 Free PMC article. Review.
-
Broadly cross-reactive human antibodies that inhibit genogroup I and II noroviruses.Nat Commun. 2021 Jul 14;12(1):4320. doi: 10.1038/s41467-021-24649-w. Nat Commun. 2021. PMID: 34262046 Free PMC article.
-
Antigenic Diversity of Human Norovirus Capsid Proteins Based on the Cross-Reactivities of Their Antisera.Pathogens. 2021 Aug 5;10(8):986. doi: 10.3390/pathogens10080986. Pathogens. 2021. PMID: 34451450 Free PMC article.
References
-
- Van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, Iritani N, Kroneman A, Vennema H, Vinjé J, White PA, Koopmans M. 2013. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill 18:8–9. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20345. - PubMed
-
- Giammanco GM, De Grazia S, Terio V, Lanave G, Catella C, Bonura F, Saporito L, Medici MC, Tummolo F, Calderaro A, Bányai K, Hansman G, Martella V. 2014. Analysis of early strains of the norovirus pandemic variant GII.4 Sydney 2012 identifies mutations in adaptive sites of the capsid protein. Virology 450–451:355–358. doi:10.1016/j.virol.2013.12.007. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

