Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B
- PMID: 25538195
- PMCID: PMC4278539
- DOI: 10.1128/mBio.02234-14
Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B
Abstract
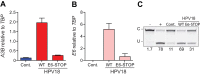
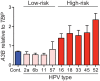
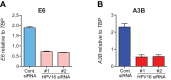
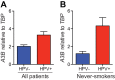
Several recent studies have converged upon the innate immune DNA cytosine deaminase APOBEC3B (A3B) as a significant source of genomic uracil lesions and mutagenesis in multiple human cancers, including those of the breast, head/neck, cervix, bladder, lung, ovary, and other tissues. A3B is upregulated in these tumor types relative to normal tissues, but the mechanism is unclear. Because A3B also has antiviral activity in multiple systems and is a member of the broader innate immune response, we tested the hypothesis that human papillomavirus (HPV) infection causes A3B upregulation. We found that A3B mRNA expression and enzymatic activity were upregulated following transfection of a high-risk HPV genome and that this effect was abrogated by inactivation of E6. Transduction experiments showed that the E6 oncoprotein alone was sufficient to cause A3B upregulation, and a panel of high-risk E6 proteins triggered higher A3B levels than did a panel of low-risk or noncancer E6 proteins. Knockdown experiments in HPV-positive cell lines showed that endogenous E6 is required for A3B upregulation. Analyses of publicly available head/neck cancer data further support this relationship, as A3B levels are higher in HPV-positive cancers than in HPV-negative cancers. Taken together with the established role for high-risk E6 in functional inactivation of TP53 and published positive correlations in breast cancer between A3B upregulation and genetic inactivation of TP53, our studies suggest a model in which high-risk HPV E6, possibly through functional inactivation of TP53, causes derepression of A3B gene transcription. This would lead to a mutator phenotype that explains the observed cytosine mutation biases in HPV-positive head/neck and cervical cancers.
Importance: The innate immune DNA cytosine deaminase APOBEC3B (A3B) accounts for a large proportion of somatic mutations in cervical and head/neck cancers, but nothing is known about the mechanism responsible for its upregulation in these tumor types. Almost all cervical carcinomas and large proportions of head/neck tumors are caused by human papillomavirus (HPV) infection. Here, we establish a mechanistic link between HPV infection and A3B upregulation. The E6 oncoprotein of high-risk, but not low-risk, HPV types triggers A3B upregulation, supporting a model in which TP53 inactivation causes a derepression of A3B gene transcription and elevated A3B enzyme levels. This virus-induced mutator phenotype provides a mechanistic explanation for A3B signature mutations observed in HPV-positive head/neck and cervical carcinomas and may also help to account for the preferential cancer predisposition caused by high-risk HPV isolates.
Copyright © 2014 Vieira et al.
Figures

Similar articles
-
Human Papillomavirus 16 E6 Upregulates APOBEC3B via the TEAD Transcription Factor.J Virol. 2017 Feb 28;91(6):e02413-16. doi: 10.1128/JVI.02413-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077648 Free PMC article.
-
Identification of APOBEC3B promoter elements responsible for activation by human papillomavirus type 16 E6.Biochem Biophys Res Commun. 2015 May 8;460(3):555-60. doi: 10.1016/j.bbrc.2015.03.068. Epub 2015 Mar 20. Biochem Biophys Res Commun. 2015. PMID: 25800874
-
Endogenous APOBEC3B overexpression characterizes HPV-positive and HPV-negative oral epithelial dysplasias and head and neck cancers.Mod Pathol. 2021 Feb;34(2):280-290. doi: 10.1038/s41379-020-0617-x. Epub 2020 Jul 6. Mod Pathol. 2021. PMID: 32632179 Free PMC article.
-
Roles of APOBEC3A and APOBEC3B in Human Papillomavirus Infection and Disease Progression.Viruses. 2017 Aug 21;9(8):233. doi: 10.3390/v9080233. Viruses. 2017. PMID: 28825669 Free PMC article. Review.
-
Post-Transcriptional Gene Regulation by HPV 16E6 and Its Host Protein Partners.Viruses. 2022 Jul 6;14(7):1483. doi: 10.3390/v14071483. Viruses. 2022. PMID: 35891463 Free PMC article. Review.
Cited by
-
Analysis of APOBEC3A/3B germline deletion polymorphism in breast, cervical and oral cancers from South India and its impact on miRNA regulation.Tumour Biol. 2016 Sep;37(9):11983-11990. doi: 10.1007/s13277-016-5064-4. Epub 2016 May 7. Tumour Biol. 2016. PMID: 27155849
-
Mutational impact of APOBEC3A and APOBEC3B in a human cell line and comparisons to breast cancer.PLoS Genet. 2023 Nov 30;19(11):e1011043. doi: 10.1371/journal.pgen.1011043. eCollection 2023 Nov. PLoS Genet. 2023. PMID: 38033156 Free PMC article.
-
Presence of human papillomavirus in breast cancer and its association with prognostic factors.Ecancermedicalscience. 2015 Jun 25;9:548. doi: 10.3332/ecancer.2015.548. eCollection 2015. Ecancermedicalscience. 2015. PMID: 26180547 Free PMC article.
-
The role of APOBEC3B in lung tumor evolution and targeted cancer therapy resistance.Nat Genet. 2024 Jan;56(1):60-73. doi: 10.1038/s41588-023-01592-8. Epub 2023 Dec 4. Nat Genet. 2024. PMID: 38049664 Free PMC article.
-
The APOBEC3B cytidine deaminase is an adenovirus restriction factor.PLoS Pathog. 2023 Feb 6;19(2):e1011156. doi: 10.1371/journal.ppat.1011156. eCollection 2023 Feb. PLoS Pathog. 2023. PMID: 36745676 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
