A Regulatory Network Involving β-Catenin, e-Cadherin, PI3k/Akt, and Slug Balances Self-Renewal and Differentiation of Human Pluripotent Stem Cells In Response to Wnt Signaling
- PMID: 25538040
- PMCID: PMC5297972
- DOI: 10.1002/stem.1944
A Regulatory Network Involving β-Catenin, e-Cadherin, PI3k/Akt, and Slug Balances Self-Renewal and Differentiation of Human Pluripotent Stem Cells In Response to Wnt Signaling
Abstract
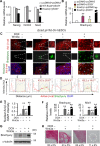
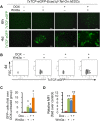
The mechanisms underlying disparate roles of the canonical Wnt signaling pathway in maintaining self-renewal or inducing differentiation and lineage specification in embryonic stem cells (ESCs) are not clear. In this study, we provide the first demonstration that self-renewal versus differentiation of human ESCs (hESCs) in response to Wnt signaling is predominantly determined by a two-layer regulatory circuit involving β-catenin, E-cadherin, PI3K/Akt, and Slug in a time-dependent manner. Short-term upregulation of β-catenin does not lead to the activation of T-cell factor (TCF)-eGFP Wnt reporter in hESCs. Instead, it enhances E-cadherin expression on the cell membrane, thereby enhancing hESC self-renewal through E-cadherin-associated PI3K/Akt signaling. Conversely, long-term Wnt activation or loss of E-cadherin intracellular β-catenin binding domain induces TCF-eGFP activity and promotes hESC differentiation through β-catenin-induced upregulation of Slug. Enhanced expression of Slug leads to a further reduction of E-cadherin that serves as a β-catenin "sink" sequestering free cytoplasmic β-catenin. The formation of such a framework reinforces hESCs to switch from a state of temporal self-renewal associated with short-term Wnt/β-catenin activation to definitive differentiation. Stem Cells 2015;33:1419-1433.
Keywords: Differentiation; E-cadherin; Human embryonic stem cell; Self-renewal; Slug; Wnt; β-Catenin.
© 2015 AlphaMed Press.
Figures

Similar articles
-
β-Catenin in pluripotency: adhering to self-renewal or Wnting to differentiate?Int Rev Cell Mol Biol. 2014;312:53-78. doi: 10.1016/B978-0-12-800178-3.00002-6. Int Rev Cell Mol Biol. 2014. PMID: 25262238 Review.
-
Perturbed differentiation of murine embryonic stem cells upon Pelota deletion due to dysregulated FOXO1/β-catenin signaling.FEBS J. 2021 May;288(10):3317-3329. doi: 10.1111/febs.15643. Epub 2020 Dec 17. FEBS J. 2021. PMID: 33245852
-
Progesterone Receptor Membrane Component 1 suppresses the p53 and Wnt/β-catenin pathways to promote human pluripotent stem cell self-renewal.Sci Rep. 2018 Feb 14;8(1):3048. doi: 10.1038/s41598-018-21322-z. Sci Rep. 2018. PMID: 29445107 Free PMC article.
-
Activation of Wnt/β-Catenin Signaling Pathway Enhances the Derivation of Buffalo (Bubalus bubalis) Embryonic Stem Cell-Like Cells.Cell Reprogram. 2020 Aug;22(4):217-225. doi: 10.1089/cell.2020.0027. Epub 2020 Jul 14. Cell Reprogram. 2020. PMID: 32673062
-
Maintaining embryonic stem cell pluripotency with Wnt signaling.Development. 2011 Oct;138(20):4341-50. doi: 10.1242/dev.066209. Epub 2011 Sep 8. Development. 2011. PMID: 21903672 Free PMC article. Review.
Cited by
-
Cdh1 functions as an oncogene by inducing self-renewal of lung cancer stem-like cells via oncogenic pathways.Int J Biol Sci. 2020 Jan 1;16(3):447-459. doi: 10.7150/ijbs.38672. eCollection 2020. Int J Biol Sci. 2020. PMID: 32015681 Free PMC article.
-
Subcellular localization of mutated β-catenins with different incidences of cis-peptide bonds at the Xaa246-P247 site in HepG2 cells.FASEB J. 2019 May;33(5):6574-6583. doi: 10.1096/fj.201801937RR. Epub 2019 Feb 26. FASEB J. 2019. PMID: 30807699 Free PMC article.
-
Mitochondrial Akt Signaling Modulated Reprogramming of Somatic Cells.Sci Rep. 2019 Jul 9;9(1):9919. doi: 10.1038/s41598-019-46359-6. Sci Rep. 2019. PMID: 31289326 Free PMC article.
-
Dual inhibition of Wnt and Yes-associated protein signaling retards the growth of triple-negative breast cancer in both mesenchymal and epithelial states.Mol Oncol. 2018 Apr;12(4):423-440. doi: 10.1002/1878-0261.12167. Epub 2018 Feb 21. Mol Oncol. 2018. PMID: 29316250 Free PMC article.
-
Phosphatase of regenerating liver-3 inhibits invasiveness and proliferation in non-small cell lung cancer by regulating the epithelial-mesenchymal transition.Oncotarget. 2016 Apr 19;7(16):21799-811. doi: 10.18632/oncotarget.7985. Oncotarget. 2016. PMID: 26967563 Free PMC article.
References
-
- Sato N, Meijer L, Skaltsounis L et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK‐3‐specific inhibitor. Nat Med 2004;10:55–63. - PubMed
-
- Singla DK, Schneider DJ, LeWinter MM et al. wnt3a but not wnt11 supports self‐renewal of embryonic stem cells. Biochem Biophys Res Commun 2006;345:789–795. - PubMed
-
- Miki T, Yasuda SY, Kahn M. Wnt/beta‐catenin signaling in embryonic stem cell self‐renewal and somatic cell reprogramming. Stem Cell Rev 2011;7:836–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

