The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor
- PMID: 25536463
- PMCID: PMC4615309
- DOI: 10.4161/19490976.2014.972241
The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor
Abstract
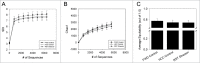
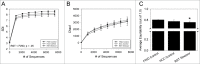
The commensal microbiota of the human gastrointestinal tract live in a largely stable community structure, assisting in host physiological and immunological functions. Changes to this structure can be injurious to the health of the host, a concept termed dysbiosis. Psychological stress is a factor that has been implicated in causing dysbiosis, and studies performed by our lab have shown that restraint stress can indeed shift the cecal microbiota structure as well as increase the severity of a colonic infection caused by Citrobacter rodentium. However, this study, like many others, have focused on fecal contents when examining the effect of dysbiosis-causing stimuli (e.g. psychological stress) upon the microbiota. Since the mucosa-associated microbiota have unique properties and functions that can act upon the host, it is important to understand how stressor exposure might affect this niche of bacteria. To begin to understand whether chronic restraint stress changes the mucosa-associated and/or luminal microbiota mice underwent 7 16-hour cycles of restraint stress, and the microbiota of both colonic tissue and fecal contents were analyzed by sequencing using next-gen bacterial tag-encoded FLX amplicon technology (bTEFAP) pyrosequencing. Both control and stress groups had significantly different mucosa-associated and luminal microbiota communities, highlighting the importance of focusing gastrointestinal community structure analysis by microbial niche. Furthermore, restraint stress was able to disrupt both the mucosa-associated and luminally-associated colonic microbiota by shifting the relative abundances of multiple groups of bacteria. Among these changes, there was a significant reduction in the immunomodulatory commensal genus Lactobacillus associated with colonic mucosa. The relative abundance of Lactobacillus spp. was not affected in the lumen. These results indicate that stressor-exposure can have distinct effects upon the colonic microbiota situated at the mucosal epithelium in comparison to the luminal-associated microbiota.
Keywords: Psychological stress; inflammation; microbiota; pyrosequencing.
Figures



Similar articles
-
Prolonged restraint stressor exposure in outbred CD-1 mice impacts microbiota, colonic inflammation, and short chain fatty acids.PLoS One. 2018 May 9;13(5):e0196961. doi: 10.1371/journal.pone.0196961. eCollection 2018. PLoS One. 2018. PMID: 29742146 Free PMC article.
-
Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium.Infect Immun. 2010 Apr;78(4):1509-19. doi: 10.1128/IAI.00862-09. Epub 2010 Feb 9. Infect Immun. 2010. PMID: 20145094 Free PMC article.
-
Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota.BMC Microbiol. 2014 Jul 15;14:189. doi: 10.1186/1471-2180-14-189. BMC Microbiol. 2014. PMID: 25028050 Free PMC article.
-
Impact of stressor exposure on the interplay between commensal microbiota and host inflammation.Gut Microbes. 2014 May-Jun;5(3):390-6. doi: 10.4161/gmic.28683. Epub 2014 Apr 1. Gut Microbes. 2014. PMID: 24690880 Free PMC article. Review.
-
The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation.Horm Behav. 2012 Aug;62(3):286-94. doi: 10.1016/j.yhbeh.2012.02.006. Epub 2012 Feb 15. Horm Behav. 2012. PMID: 22366706 Review.
Cited by
-
Proteomics and Metaproteomics Add Functional, Taxonomic and Biomass Dimensions to Modeling the Ecosystem at the Mucosal-luminal Interface.Mol Cell Proteomics. 2020 Sep;19(9):1409-1417. doi: 10.1074/mcp.R120.002051. Epub 2020 Jun 24. Mol Cell Proteomics. 2020. PMID: 32581040 Free PMC article. Review.
-
Effect of chronic stress on gel-forming mucins in the small intestine of BALB/c mice.J Med Life. 2024 Mar;17(3):326-333. doi: 10.25122/jml-2023-0473. J Med Life. 2024. PMID: 39044931 Free PMC article.
-
Radiation enteropathy-related depression: A neglectable course of disease by gut bacterial dysbiosis.Cancer Med. 2024 Feb;13(4):e6865. doi: 10.1002/cam4.6865. Cancer Med. 2024. PMID: 38457257 Free PMC article. Review.
-
The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment.Brain Behav Immun Health. 2022 Oct 29;26:100541. doi: 10.1016/j.bbih.2022.100541. eCollection 2022 Dec. Brain Behav Immun Health. 2022. PMID: 36536630 Free PMC article.
-
A Microbiome-Driven Approach to Combating Depression During the COVID-19 Pandemic.Front Nutr. 2021 Aug 24;8:672390. doi: 10.3389/fnut.2021.672390. eCollection 2021. Front Nutr. 2021. PMID: 34504858 Free PMC article.
References
-
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012; 9:286-94; PMID:22392290; http://dx.doi.org/10.1038/nrgastro.2012.32 - DOI - PubMed
-
- Holzer P. Efferent-like roles of afferent neurons in the gut: Blood flow regulation and tissue protection. Autonom Neurosci: Basic Clin 2006; 125:70-5; http://dx.doi.org/10.1016/j.autneu.2006.01.004 - DOI - PMC - PubMed
-
- de Jonge WJ. The Gut's Little Brain in Control of Intestinal Immunity. ISRN Gastroenterol 2013; 2013:630159; PMID:23691339; http://dx.doi.org/10.1155/2013/630159 - DOI - PMC - PubMed
-
- Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol 2010; 105:1994-2002; PMID:20372115; http://dx.doi.org/10.1038/ajg.2010.140 - DOI - PubMed
-
- Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 1992; 33:825-30; PMID:1624167; http://dx.doi.org/10.1136/gut.33.6.825 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
