Wild-type and specific mutant androgen receptor mediates transcription via 17β-estradiol in sex hormone-sensitive cancer cells
- PMID: 25536295
- PMCID: PMC6680109
- DOI: 10.1002/jcp.24906
Wild-type and specific mutant androgen receptor mediates transcription via 17β-estradiol in sex hormone-sensitive cancer cells
Abstract
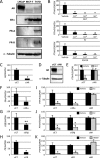
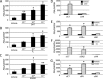
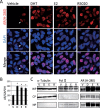
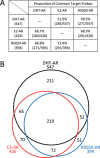
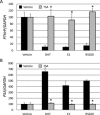
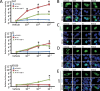
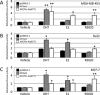
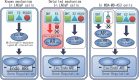
We previously encountered regulatory processes wherein dihydrotestosterone (DHT) exerted its inhibitory effect on parathyroid hormone-related protein (PTHrP) gene repression through the estrogen receptor (ER)α, but not the androgen receptor (AR), in breast cancer MCF-7 cells. Here, we investigated whether such aberrant ligand-nuclear receptor (NR) interaction is present in prostate cancer LNCaP cells. First, we confirmed that LNCaP cells expressed large amounts of AR at negligible levels of ERα/β or progesterone receptor. Both suppression of PTHrP and activation of prostate-specific antigen genes were observed after independent administration of 17β-estradiol (E2), DHT, or R5020. Consistent with the notion that the LNCaP AR lost its ligand specificity due to a mutation (Thr-Ala877), experiments with siRNA targeting the respective NR revealed that the AR monopolized the role of the mediator of shared hormone-dependent regulation, which was invariably associated with nuclear translocation of this mutant AR. Microarray analysis of gene regulation by DHT, E2, or R5020 disclosed that more than half of the genes downstream of the AR (Thr-Ala877) overlapped in the LNCaP cells. Of particular interest, we realized that the AR (wild-type [wt]) and AR (Thr-Ala877) were equally responsible for the E2-AR interactions. Fluorescence microscopy experiments demonstrated that both EGFP-AR (wt) and EGFP-AR (Thr-Ala877) were exclusively localized within the nucleus after E2 or DHT treatment. Furthermore, reporter assays revealed that some other cancer cells exhibited aberrant E2-AR (wt) signaling similar to that in the LNCaP cells. We herein postulate the presence of entangled interactions between wt AR and E2 in certain hormone-sensitive cancer cells.
© 2014 Wiley Periodicals, Inc.
Figures
Similar articles
-
Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence.Cancer Res. 2004 Oct 1;64(19):7156-68. doi: 10.1158/0008-5472.CAN-04-1121. Cancer Res. 2004. PMID: 15466214
-
Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells.Am J Physiol Endocrinol Metab. 2005 Mar;288(3):E573-84. doi: 10.1152/ajpendo.00454.2004. Epub 2004 Nov 9. Am J Physiol Endocrinol Metab. 2005. PMID: 15536203
-
TGF-beta signaling and androgen receptor status determine apoptotic cross-talk in human prostate cancer cells.Prostate. 2008 Feb 15;68(3):287-95. doi: 10.1002/pros.20698. Prostate. 2008. PMID: 18163430
-
Androgen-receptor gene structure and function in prostate cancer.World J Urol. 1996;14(5):329-37. doi: 10.1007/BF00184606. World J Urol. 1996. PMID: 8912473 Review.
-
Androgen receptor and estrogen receptor variants in prostate and breast cancers.J Steroid Biochem Mol Biol. 2024 Jul;241:106522. doi: 10.1016/j.jsbmb.2024.106522. Epub 2024 Apr 17. J Steroid Biochem Mol Biol. 2024. PMID: 38641298 Review.
Cited by
-
Role of adipocyte browning in prostate and breast tumor microenvironment.Tzu Chi Med J. 2022 Jun 27;34(4):359-366. doi: 10.4103/tcmj.tcmj_62_22. eCollection 2022 Oct-Dec. Tzu Chi Med J. 2022. PMID: 36578640 Free PMC article. Review.
-
Solving the Puzzle: What Is the Role of Progestogens in Neovascularization?Biomolecules. 2021 Nov 12;11(11):1686. doi: 10.3390/biom11111686. Biomolecules. 2021. PMID: 34827682 Free PMC article. Review.
-
Intrinsic ubiquitin E3 ligase activity of histone acetyltransferase Hbo1 for estrogen receptor α.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):498-510. doi: 10.2183/pjab.93.030. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28769019 Free PMC article.
-
Novel estrogen-responsive genes (ERGs) for the evaluation of estrogenic activity.PLoS One. 2022 Aug 17;17(8):e0273164. doi: 10.1371/journal.pone.0273164. eCollection 2022. PLoS One. 2022. PMID: 35976950 Free PMC article.
-
Without 1α-hydroxylation, the gene expression profile of 25(OH)D3 treatment overlaps deeply with that of 1,25(OH)2D3 in prostate cancer cells.Sci Rep. 2018 Jun 13;8(1):9024. doi: 10.1038/s41598-018-27441-x. Sci Rep. 2018. PMID: 29899561 Free PMC article.
References
-
- Ahlstrom M, Pekkinen M, Lamberg‐Allardt C. 2009. Dexamethasone downregulates the expression of parathyroid hormone‐related protein (PTHrP) in mesenchymal stem cells. Steroids 74:277–282. - PubMed
-
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. 1987. Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science 237:268–275. - PubMed
-
- Asadi F, Kukreja S. 2005. Parathyroid hormone‐related protein in prostate cancer. Crit Rev Eukaryot Gene Expr 15:15–28. - PubMed
-
- Bektic J, Berger AP, Pfeil K, Dobler G, Bartsch G, Klocker H. 2004. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen‐dependent LNCaP cells is mediated by estrogen receptor beta. Eur Urol 45:245–251; discussion251. - PubMed
-
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

