Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation
- PMID: 25531312
- PMCID: PMC4274750
- DOI: 10.1038/ncomms6780
Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation
Abstract
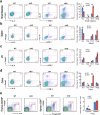
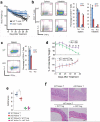
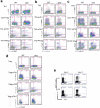
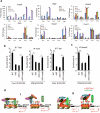
Epigenetic factors have been implicated in the regulation of CD4(+) T-cell differentiation. Jmjd3 plays a role in many biological processes, but its in vivo function in T-cell differentiation remains unknown. Here we report that Jmjd3 ablation promotes CD4(+) T-cell differentiation into Th2 and Th17 cells in the small intestine and colon, and inhibits T-cell differentiation into Th1 cells under different cytokine-polarizing conditions and in a Th1-dependent colitis model. Jmjd3 deficiency also restrains the plasticity of the conversion of Th2, Th17 or Treg cells to Th1 cells. The skewing of T-cell differentiation is concomitant with changes in the expression of key transcription factors and cytokines. H3K27me3 and H3K4me3 levels in Jmjd3-deficient cells are correlated with altered gene expression through interactions with specific transcription factors. Our results identify Jmjd3 as an epigenetic factor in T-cell differentiation via changes in histone methylation and target gene expression.
Figures




Similar articles
-
The histone H3 lysine-27 demethylase Jmjd3 plays a critical role in specific regulation of Th17 cell differentiation.J Mol Cell Biol. 2015 Dec;7(6):505-16. doi: 10.1093/jmcb/mjv022. Epub 2015 Apr 3. J Mol Cell Biol. 2015. PMID: 25840993
-
Histone H3 Lysine 27 demethylases Jmjd3 and Utx are required for T-cell differentiation.Nat Commun. 2015 Sep 2;6:8152. doi: 10.1038/ncomms9152. Nat Commun. 2015. PMID: 26328764 Free PMC article.
-
Histone demethylase JMJD3 regulates CD11a expression through changes in histone H3K27 tri-methylation levels in CD4+ T cells of patients with systemic lupus erythematosus.Oncotarget. 2017 Jul 25;8(30):48938-48947. doi: 10.18632/oncotarget.16894. Oncotarget. 2017. PMID: 28430662 Free PMC article.
-
Histone demethylase Jumonji D3 (JMJD3/KDM6B) at the nexus of epigenetic regulation of inflammation and the aging process.J Mol Med (Berl). 2014 Oct;92(10):1035-43. doi: 10.1007/s00109-014-1182-x. Epub 2014 Jun 14. J Mol Med (Berl). 2014. PMID: 24925089 Review.
-
The role and prospect of JMJD3 in stem cells and cancer.Biomed Pharmacother. 2019 Oct;118:109384. doi: 10.1016/j.biopha.2019.109384. Epub 2019 Sep 6. Biomed Pharmacother. 2019. PMID: 31545292 Review.
Cited by
-
Targeting Interleukin-17 as a Novel Treatment Option for Fibrotic Diseases.J Clin Med. 2023 Dec 27;13(1):164. doi: 10.3390/jcm13010164. J Clin Med. 2023. PMID: 38202170 Free PMC article. Review.
-
Histone demethylases in physiology and cancer: a tale of two enzymes, JMJD3 and UTX.Curr Opin Genet Dev. 2016 Feb;36:59-67. doi: 10.1016/j.gde.2016.03.010. Epub 2016 May 3. Curr Opin Genet Dev. 2016. PMID: 27151432 Free PMC article. Review.
-
DNMT and HDAC inhibitors together abrogate endotoxemia mediated macrophage death by STAT3-JMJD3 signaling.Int J Biochem Cell Biol. 2018 Sep;102:117-127. doi: 10.1016/j.biocel.2018.07.002. Epub 2018 Jul 24. Int J Biochem Cell Biol. 2018. PMID: 30010012 Free PMC article.
-
Histone demethylase PHF2 activates CREB and promotes memory consolidation.EMBO Rep. 2019 Sep;20(9):e45907. doi: 10.15252/embr.201845907. Epub 2019 Jul 30. EMBO Rep. 2019. PMID: 31359606 Free PMC article.
-
JMJD3 facilitates C/EBPβ-centered transcriptional program to exert oncorepressor activity in AML.Nat Commun. 2018 Aug 22;9(1):3369. doi: 10.1038/s41467-018-05548-z. Nat Commun. 2018. PMID: 30135572 Free PMC article.
References
-
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. - PubMed
-
- Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. - PubMed
-
- Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

