Host endoplasmic reticulum COPII proteins control cell-to-cell spread of the bacterial pathogen Listeria monocytogenes
- PMID: 25529574
- PMCID: PMC4656193
- DOI: 10.1111/cmi.12409
Host endoplasmic reticulum COPII proteins control cell-to-cell spread of the bacterial pathogen Listeria monocytogenes
Abstract
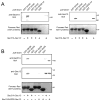
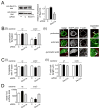
Listeria monocytogenes is a food-borne pathogen that uses actin-dependent motility to spread between human cells. Cell-to-cell spread involves the formation by motile bacteria of plasma membrane-derived structures termed 'protrusions'. In cultured enterocytes, the secreted Listeria protein InlC promotes protrusion formation by binding and inhibiting the human scaffolding protein Tuba. Here we demonstrate that protrusions are controlled by human COPII components that direct trafficking from the endoplasmic reticulum. Co-precipitation experiments indicated that the COPII proteins Sec31A and Sec13 interact directly with a Src homology 3 domain in Tuba. This interaction was antagonized by InlC. Depletion of Sec31A or Sec13 restored normal protrusion formation to a Listeria mutant lacking inlC, without affecting spread of wild-type bacteria. Genetic impairment of the COPII component Sar1 or treatment of cells with brefeldin A affected protrusions similarly to Sec31A or Sec13 depletion. These findings indicated that InlC relieves a host-mediated restriction of Listeria spread otherwise imposed by COPII. Inhibition of Sec31A, Sec13 or Sar1 or brefeldin A treatment also perturbed the structure of cell-cell junctions. Collectively, these findings demonstrate an important role for COPII in controlling Listeria spread. We propose that COPII may act by delivering host proteins that generate tension at cell junctions.
© 2014 John Wiley & Sons Ltd.
Figures

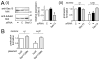
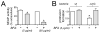

Similar articles
-
Bacterial spread from cell to cell: beyond actin-based motility.Trends Microbiol. 2015 Sep;23(9):558-66. doi: 10.1016/j.tim.2015.04.010. Epub 2015 May 25. Trends Microbiol. 2015. PMID: 26021574 Free PMC article. Review.
-
The host GTPase Dynamin 2 modulates apical junction structure to control cell-to-cell spread of Listeria monocytogenes.Infect Immun. 2024 Oct 15;92(10):e0013624. doi: 10.1128/iai.00136-24. Epub 2024 Aug 12. Infect Immun. 2024. PMID: 39133017
-
Molecular mechanism of protrusion formation during cell-to-cell spread of Listeria.Front Cell Infect Microbiol. 2014 Feb 21;4:21. doi: 10.3389/fcimb.2014.00021. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24600591 Free PMC article. Review.
-
Listeria monocytogenes exploits host exocytosis to promote cell-to-cell spread.Proc Natl Acad Sci U S A. 2020 Feb 18;117(7):3789-3796. doi: 10.1073/pnas.1916676117. Epub 2020 Feb 3. Proc Natl Acad Sci U S A. 2020. PMID: 32015134 Free PMC article.
-
Listeria monocytogenes antagonizes the human GTPase Cdc42 to promote bacterial spread.Cell Microbiol. 2014 Jul;16(7):1068-79. doi: 10.1111/cmi.12260. Epub 2014 Jan 24. Cell Microbiol. 2014. PMID: 24405483 Free PMC article.
Cited by
-
Bacterial spread from cell to cell: beyond actin-based motility.Trends Microbiol. 2015 Sep;23(9):558-66. doi: 10.1016/j.tim.2015.04.010. Epub 2015 May 25. Trends Microbiol. 2015. PMID: 26021574 Free PMC article. Review.
-
A targeted approach to investigating immune genes of an iconic Australian marsupial.Mol Ecol. 2022 Jun;31(12):3286-3303. doi: 10.1111/mec.16493. Epub 2022 May 17. Mol Ecol. 2022. PMID: 35510793 Free PMC article.
-
The host GTPase Dynamin 2 modulates apical junction structure to control cell-to-cell spread of Listeria monocytogenes.Infect Immun. 2024 Oct 15;92(10):e0013624. doi: 10.1128/iai.00136-24. Epub 2024 Aug 12. Infect Immun. 2024. PMID: 39133017
-
Actin-based motility and cell-to-cell spread of bacterial pathogens.Curr Opin Microbiol. 2017 Feb;35:48-57. doi: 10.1016/j.mib.2016.11.007. Epub 2016 Dec 19. Curr Opin Microbiol. 2017. PMID: 27997855 Free PMC article. Review.
-
The Host Scaffolding Protein Filamin A and the Exocyst Complex Control Exocytosis during InlB-Mediated Entry of Listeria monocytogenes.Infect Immun. 2018 Dec 19;87(1):e00689-18. doi: 10.1128/IAI.00689-18. Print 2019 Jan. Infect Immun. 2018. PMID: 30348826 Free PMC article.
References
-
- Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

