Mitochondrial p32 is upregulated in Myc expressing brain cancers and mediates glutamine addiction
- PMID: 25528767
- PMCID: PMC4359224
- DOI: 10.18632/oncotarget.2708
Mitochondrial p32 is upregulated in Myc expressing brain cancers and mediates glutamine addiction
Abstract
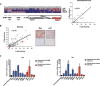
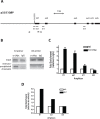
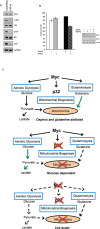
Metabolic reprogramming is a key feature of tumorigenesis that is controlled by oncogenes. Enhanced utilization of glucose and glutamine are the best-established hallmarks of tumor metabolism. The oncogene c-Myc is one of the major players responsible for this metabolic alteration. However, the molecular mechanisms involved in Myc-induced metabolic reprogramming are not well defined. Here we identify p32, a mitochondrial protein known to play a role in the expression of mitochondrial respiratory chain complexes, as a critical player in Myc-induced glutamine addiction. We show that p32 is a direct transcriptional target of Myc and that high level of Myc in malignant brain cancers correlates with high expression of p32. Attenuation of p32 expression reduced growth rate of glioma cells expressing Myc and impaired tumor formation in vivo. Loss of p32 in glutamine addicted glioma cells induced resistance to glutamine deprivation and imparted sensitivity to glucose withdrawal. Finally, we provide evidence that p32 expression contributes to Myc-induced glutamine addiction of cancer cells. Our findings suggest that Myc promotes the expression of p32, which is required to maintain sufficient respiratory capacity to sustain glutamine metabolism in Myc transformed cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Expression of far upstream element (FUSE) binding protein 1 in human glioma is correlated with c-Myc and cell proliferation.Mol Carcinog. 2015 May;54(5):405-15. doi: 10.1002/mc.22114. Epub 2013 Dec 17. Mol Carcinog. 2015. PMID: 24347226
-
SIRT4 protein suppresses tumor formation in genetic models of Myc-induced B cell lymphoma.J Biol Chem. 2014 Feb 14;289(7):4135-44. doi: 10.1074/jbc.M113.525949. Epub 2013 Dec 24. J Biol Chem. 2014. PMID: 24368766 Free PMC article.
-
Myc promotes glutaminolysis in human neuroblastoma through direct activation of glutaminase 2.Oncotarget. 2015 Dec 1;6(38):40655-66. doi: 10.18632/oncotarget.5821. Oncotarget. 2015. PMID: 26528759 Free PMC article.
-
Impact of MYC in regulation of tumor cell metabolism.Biochim Biophys Acta. 2015 May;1849(5):563-9. doi: 10.1016/j.bbagrm.2014.07.004. Epub 2014 Jul 17. Biochim Biophys Acta. 2015. PMID: 25038584 Review.
-
Therapeutic targeting of Myc-reprogrammed cancer cell metabolism.Cold Spring Harb Symp Quant Biol. 2011;76:369-74. doi: 10.1101/sqb.2011.76.011296. Epub 2011 Sep 29. Cold Spring Harb Symp Quant Biol. 2011. PMID: 21960526 Review.
Cited by
-
The Expression Pattern of p32 in Sheep Muscle and Its Role in Differentiation, Cell Proliferation, and Apoptosis of Myoblasts.Int J Mol Sci. 2019 Oct 18;20(20):5161. doi: 10.3390/ijms20205161. Int J Mol Sci. 2019. PMID: 31635221 Free PMC article.
-
The oncogenic role of SAMMSON lncRNA in tumorigenesis: A comprehensive review with especial focus on melanoma.J Cell Mol Med. 2023 Dec;27(24):3966-3973. doi: 10.1111/jcmm.17978. Epub 2023 Sep 29. J Cell Mol Med. 2023. PMID: 37772815 Free PMC article. Review.
-
A novel small molecule inhibitor of p32 mitochondrial protein overexpressed in glioma.J Transl Med. 2017 Oct 18;15(1):210. doi: 10.1186/s12967-017-1312-7. J Transl Med. 2017. PMID: 29047383 Free PMC article.
-
In Silico and In Vivo Studies of a Tumor-Penetrating and Interfering Peptide with Antitumoral Effect on Xenograft Models of Breast Cancer.Pharmaceutics. 2023 Apr 7;15(4):1180. doi: 10.3390/pharmaceutics15041180. Pharmaceutics. 2023. PMID: 37111665 Free PMC article.
-
Small Molecule Inhibitors of Arylamine N-Acetyltransferase 1 Attenuate Cellular Respiration.ACS Pharmacol Transl Sci. 2024 Jul 17;7(8):2326-2332. doi: 10.1021/acsptsci.4c00282. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144569
References
-
- Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Funes JM, Quintero M, Henderson S, Martinez D, Qureshi U, Westwood C, Clements MO, Bourboulia D, Pedley RB, Moncada S, Boshoff C. Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc Natl Acad Sci U S A. 2007;104:6223–6228. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

