Rab11a is required for apical protein localisation in the intestine
- PMID: 25527643
- PMCID: PMC4295169
- DOI: 10.1242/bio.20148532
Rab11a is required for apical protein localisation in the intestine
Abstract
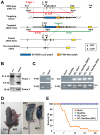
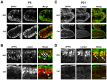
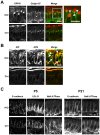
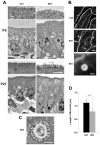
The small GTPase Rab11 plays an important role in the recycling of proteins to the plasma membrane as well as in polarised transport in epithelial cells and neurons. We generated conditional knockout mice deficient in Rab11a. Rab11a-deficient mice are embryonic lethal, and brain-specific Rab11a knockout mice show no overt abnormalities in brain architecture. In contrast, intestine-specific Rab11a knockout mice begin dying approximately 1 week after birth. Apical proteins in the intestines of knockout mice accumulate in the cytoplasm and mislocalise to the basolateral plasma membrane, whereas the localisation of basolateral proteins is unaffected. Shorter microvilli and microvillus inclusion bodies are also observed in the knockout mice. Elevation of a serum starvation marker was also observed, likely caused by the mislocalisation of apical proteins and reduced nutrient uptake. In addition, Rab8a is mislocalised in Rab11a knockout mice. Conversely, Rab11a is mislocalised in Rab8a knockout mice and in a microvillus atrophy patient, which has a mutation in the myosin Vb gene. Our data show an essential role for Rab11a in the localisation of apical proteins in the intestine and demonstrate functional relationships between Rab11a, Rab8a and myosin Vb in vivo.
Keywords: Apical membrane; Brain; Cell polarity; Intestine; Knockout mouse; Rab11a.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures

Similar articles
-
Intestinal epithelial cell polarity defects in disease: lessons from microvillus inclusion disease.Dis Model Mech. 2018 Feb 13;11(2):dmm031088. doi: 10.1242/dmm.031088. Dis Model Mech. 2018. PMID: 29590640 Free PMC article. Review.
-
Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy.J Cell Sci. 2017 Aug 1;130(15):2491-2505. doi: 10.1242/jcs.201897. Epub 2017 Jun 8. J Cell Sci. 2017. PMID: 28596241 Free PMC article.
-
Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes.J Cell Sci. 2015 Apr 15;128(8):1617-26. doi: 10.1242/jcs.163303. Epub 2015 Feb 11. J Cell Sci. 2015. PMID: 25673875 Free PMC article.
-
An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12408-13. doi: 10.1073/pnas.1516672112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392529 Free PMC article.
-
Trafficking Ion Transporters to the Apical Membrane of Polarized Intestinal Enterocytes.Cold Spring Harb Perspect Biol. 2018 Jan 2;10(1):a027979. doi: 10.1101/cshperspect.a027979. Cold Spring Harb Perspect Biol. 2018. PMID: 28264818 Free PMC article. Review.
Cited by
-
A V0-ATPase-dependent apical trafficking pathway maintains the polarity of the intestinal absorptive membrane.Development. 2019 Jun 5;146(11):dev174508. doi: 10.1242/dev.174508. Development. 2019. PMID: 31110027 Free PMC article.
-
Myo5b knockout mice as a model of microvillus inclusion disease.Sci Rep. 2015 Jul 23;5:12312. doi: 10.1038/srep12312. Sci Rep. 2015. PMID: 26201991 Free PMC article.
-
Recycling endosomes.Curr Opin Cell Biol. 2015 Aug;35:117-22. doi: 10.1016/j.ceb.2015.04.018. Epub 2015 May 27. Curr Opin Cell Biol. 2015. PMID: 26022676 Free PMC article. Review.
-
Comprehensive knockout analysis of the Rab family GTPases in epithelial cells.J Cell Biol. 2019 Jun 3;218(6):2035-2050. doi: 10.1083/jcb.201810134. Epub 2019 May 9. J Cell Biol. 2019. PMID: 31072826 Free PMC article.
-
Intestinal epithelial cell polarity defects in disease: lessons from microvillus inclusion disease.Dis Model Mech. 2018 Feb 13;11(2):dmm031088. doi: 10.1242/dmm.031088. Dis Model Mech. 2018. PMID: 29590640 Free PMC article. Review.
References
-
- Casanova J. E., Wang X., Kumar R., Bhartur S. G., Navarre J., Woodrum J. E., Altschuler Y., Ray G. S., Goldenring J. R. (1999). Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 47–61 10.1091/mbc.10.1.47 - DOI - PMC - PubMed
-
- Duman J. G., Tyagarajan K., Kolsi M. S., Moore H. P., Forte J. G. (1999). Expression of rab11a N124I in gastric parietal cells inhibits stimulatory recruitment of the H+-K+-ATPase. Am. J. Physiol. 277, C361–C372. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

