Isoform-specific functions of Mud/NuMA mediate binucleation of Drosophila male accessory gland cells
- PMID: 25527079
- PMCID: PMC4300151
- DOI: 10.1186/s12861-014-0046-5
Isoform-specific functions of Mud/NuMA mediate binucleation of Drosophila male accessory gland cells
Abstract
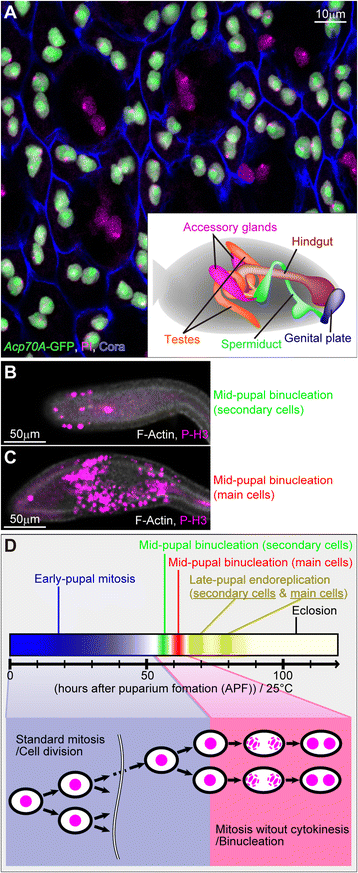
Background: In standard cell division, the cells undergo karyokinesis and then cytokinesis. Some cells, however, such as cardiomyocytes and hepatocytes, can produce binucleate cells by going through mitosis without cytokinesis. This cytokinesis skipping is thought to be due to the inhibition of cytokinesis machinery such as the central spindle or the contractile ring, but the mechanisms regulating it are unclear. We investigated them by characterizing the binucleation event during development of the Drosophila male accessory gland, in which all cells are binucleate.
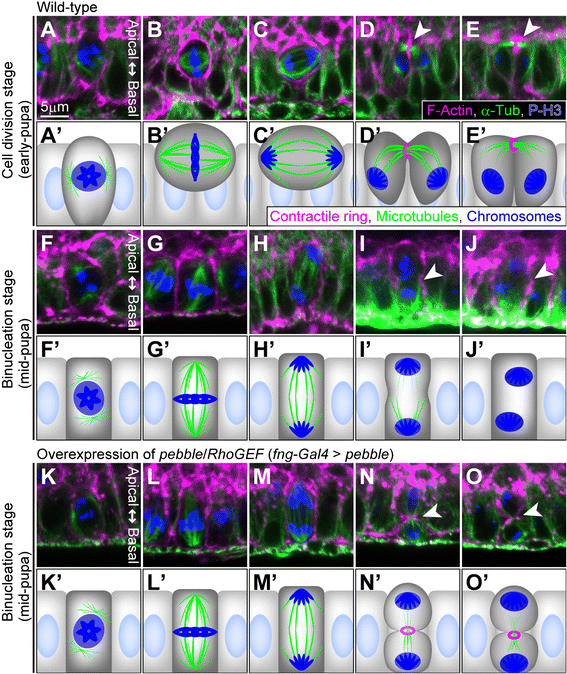
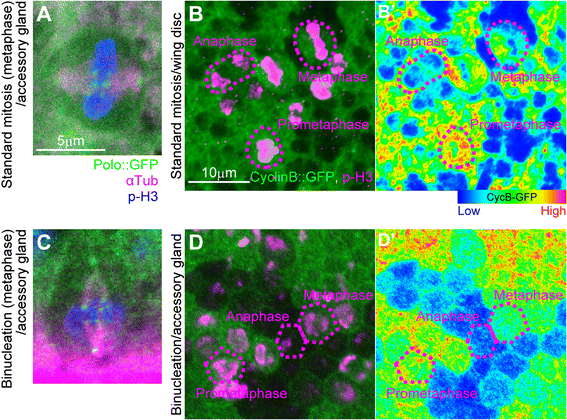
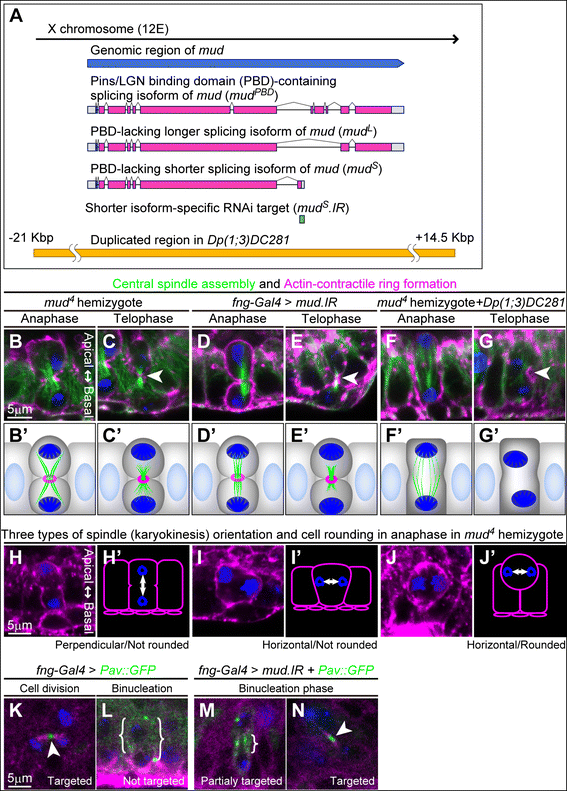
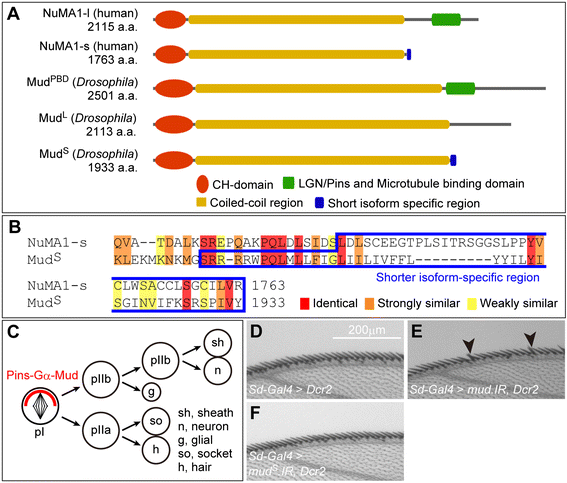
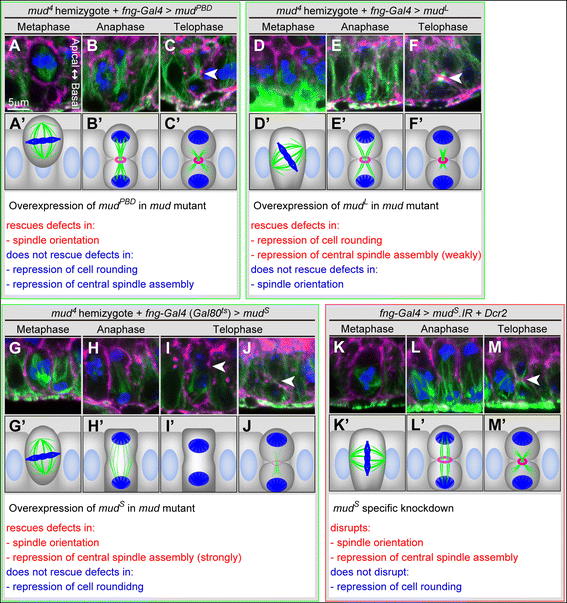
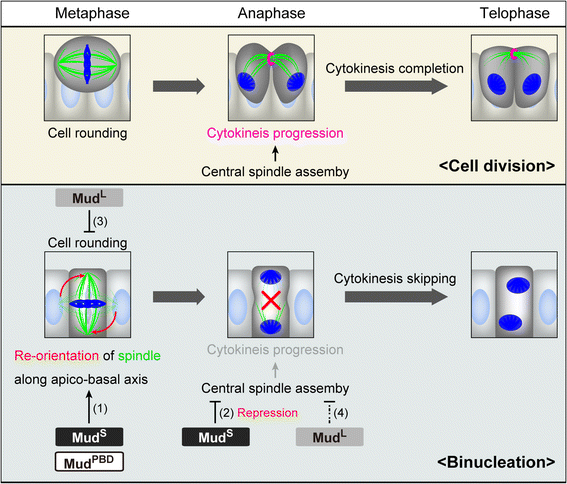
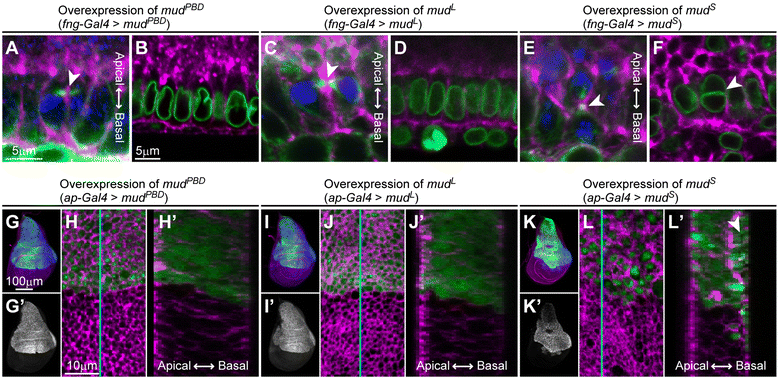
Results: The accessory gland cells arrested the cell cycle at 50 hours after puparium formation (APF) and in the middle of the pupal stage stopped proliferating for 5 hours. They then restarted the cell cycle and at 55 hours APF entered the M-phase synchronously. At this stage, accessory gland cells binucleated by mitosis without cytokinesis. Binucleating cells displayed the standard karyokinesis progression but also showed unusual features such as a non-round shape, spindle orientation along the apico-basal axis, and poor assembly of the central spindle. Mud, a Drosophila homolog of NuMA, regulated the processes responsible for these three features, the classical isoform Mud(PBD) and the two newly characterized isoforms Mud(L) and Mud(S) regulated them differently: Mud(L) repressed cell rounding, Mud(PBD) and Mud(S) oriented the spindle along the apico-basal axis, and Mud(S) and Mud(L) repressed central spindle assembly. Importantly, overexpression of Mud(S) induced binucleation even in standard proliferating cells such as those in imaginal discs.
Conclusions: We characterized the binucleation in the Drosophila male accessory gland and examined mechanisms that regulated unusual morphologies of binucleating cells. We demonstrated that Mud, a microtubule binding protein regulating spindle orientation, was involved in this binucleation. We suggest that atypical functions exerted by three structurally different isoforms of Mud regulate cell rounding, spindle orientation and central spindle assembly in binucleation. We also propose that Mud(S) is a key regulator triggering cytokinesis skipping in binucleation processes.
Figures
Similar articles
-
The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts.Nat Cell Biol. 2006 Jun;8(6):594-600. doi: 10.1038/ncb1412. Epub 2006 Apr 30. Nat Cell Biol. 2006. PMID: 16648843
-
Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization.Nat Cell Biol. 2006 Jun;8(6):586-93. doi: 10.1038/ncb1409. Epub 2006 Apr 30. Nat Cell Biol. 2006. PMID: 16648846
-
The Drosophila NuMA Homolog Mud regulates spindle orientation in asymmetric cell division.Dev Cell. 2006 Jun;10(6):731-42. doi: 10.1016/j.devcel.2006.05.005. Dev Cell. 2006. PMID: 16740476
-
Epithelial polarity and spindle orientation: intersecting pathways.Philos Trans R Soc Lond B Biol Sci. 2013 Sep 23;368(1629):20130291. doi: 10.1098/rstb.2013.0291. Print 2013. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24062590 Free PMC article. Review.
-
Relationships between the central spindle and the contractile ring during cytokinesis in animal cells.Microsc Res Tech. 2000 Apr 15;49(2):202-8. doi: 10.1002/(SICI)1097-0029(20000415)49:2<202::AID-JEMT13>3.0.CO;2-8. Microsc Res Tech. 2000. PMID: 10816260 Review.
Cited by
-
Cell-intrinsic and -extrinsic mechanisms promote cell-type-specific cytokinetic diversity.Elife. 2018 Jul 20;7:e36204. doi: 10.7554/eLife.36204. Elife. 2018. PMID: 30028292 Free PMC article.
-
Polyploid Superficial Cells that Maintain the Urothelial Barrier Are Produced via Incomplete Cytokinesis and Endoreplication.Cell Rep. 2018 Oct 9;25(2):464-477.e4. doi: 10.1016/j.celrep.2018.09.042. Cell Rep. 2018. PMID: 30304685 Free PMC article.
-
Cell cycle variants during Drosophila male accessory gland development.G3 (Bethesda). 2024 Jul 8;14(7):jkae089. doi: 10.1093/g3journal/jkae089. G3 (Bethesda). 2024. PMID: 38683731 Free PMC article.
-
A new level of plasticity: Drosophila smooth-like testes muscles compensate failure of myoblast fusion.Development. 2016 Jan 15;143(2):329-38. doi: 10.1242/dev.126730. Epub 2015 Dec 10. Development. 2016. PMID: 26657767 Free PMC article.
-
Developmental expression patterns of toolkit genes in male accessory gland of Drosophila parallels those of mammalian prostate.Biol Open. 2021 Aug 15;10(8):bio058722. doi: 10.1242/bio.058722. Epub 2021 Aug 17. Biol Open. 2021. PMID: 34342345 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

